
Exhibit 99.1

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside NASDAQ : CAPR Corporate & Investor Presentation March 2020

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Forward - Looking Statements 2 Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor's product candidates ; the initiation, conduct, size, timing and results of discovery efforts and clinical trials ; the pace of enrollment of clinical trials ; plans regarding regulatory filings, future research and clinical trials ; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market ; plans regarding current and future collaborative activities and the ownership of commercial rights ; scope, duration, validity and enforceability of intellectual property rights ; future royalty streams, revenue projections ; expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings, and any other statements about Capricor's management team's future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2018 as filed with the Securities and Exchange Commission on March 29 , 2019 , and as amended by its Amendment No . 1 to Annual Report on Form 10 - K/A filed with the Securities and Exchange Commission on April 1 , 2019 , in its Quarterly Report on Form 10 - Q for the quarterly period ended September 30 , 2019 , as filed with the Securities and Exchange Commission on November 8 , 2019 , and in its Registration Statement on Form S - 1 as filed with the Securities and Exchange Commission on December 5 , 2019 which was declared effective by the Securities and Exchange Commission on December 17 , 2019 , and the prospectus contained therein, together with any amendments and supplements thereto . All forward - looking statements in this press release are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward - looking statements . CAP - 1002 is an Investigational New Drug and is not approved for any indications . CAP - 2003 has not yet been approved for clinical investigation .
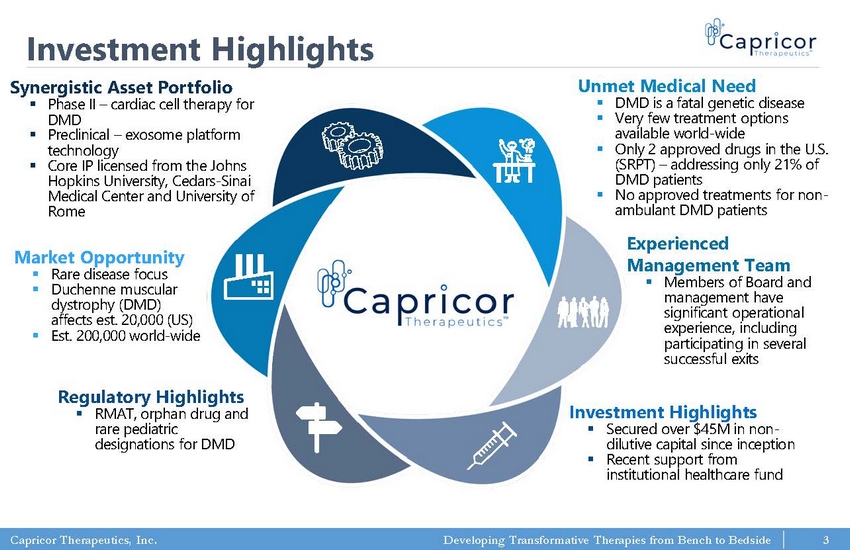
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 3 Investment Highlights Synergistic Asset Portfolio ▪ Phase II – cardiac cell therapy for DMD ▪ Preclinical – exosome platform technology ▪ Core IP licensed from the Johns Hopkins University, Cedars - Sinai Medical Center and University of Rome Regulatory Highlights ▪ RMAT, orphan drug and rare pediatric designations for DMD Experienced Management Team ▪ Members of Board and management have significant operational experience, including participating in several successful exits Unmet Medical Need ▪ DMD is a fatal genetic disease ▪ Very few treatment options available world - wide ▪ Only 2 approved drugs in the U.S. (SRPT) – addressing only 21% of DMD patients ▪ No approved treatments for non - ambulant DMD patients Market Opportunity ▪ Rare disease focus ▪ Duchenne muscular dystrophy (DMD) affects est. 20,000 (US) ▪ Est. 200,000 world - wide Investment Highlights ▪ Secured over $45M in non - dilutive capital since inception ▪ Recent support from institutional healthcare fund
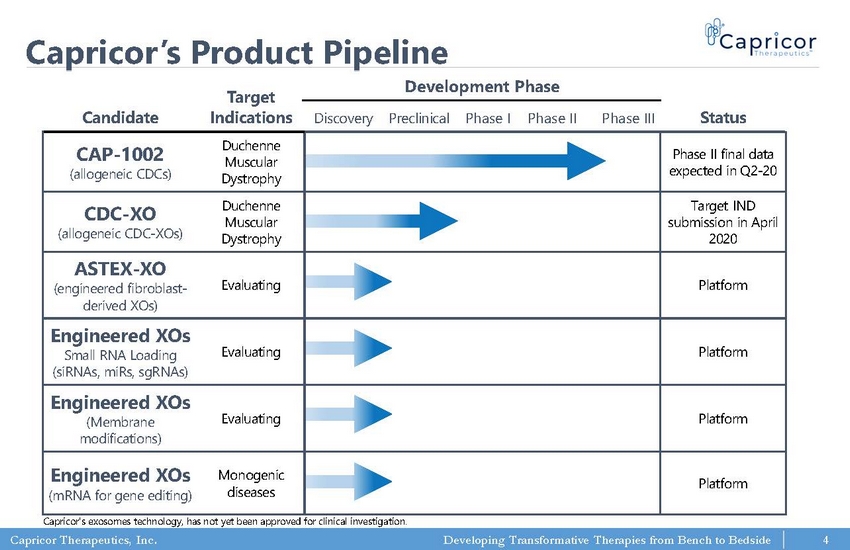
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s Product Pipeline 4 Candidate Target Indications Development Phase Status Discovery Preclinica l Phase I Phase II Phase III CAP - 1002 (allogeneic CDCs) Duchenne Muscular Dystrophy Phase II final data expected in Q2 - 20 CDC - XO (allogeneic CDC - XOs) Duchenne Muscular Dystrophy Target IND submission in April 2020 ASTEX - XO (engineered fibroblast - derived XOs) Evaluating Platform Engineered XOs Small RNA Loading (siRNAs, miRs , sgRNAs) Evaluating Platform Engineered XOs (Membrane modifications) Evaluating Platform Engineered XOs Monogenic Platform Capricor's exosomes technology, has not yet been approved for clinical investigation.
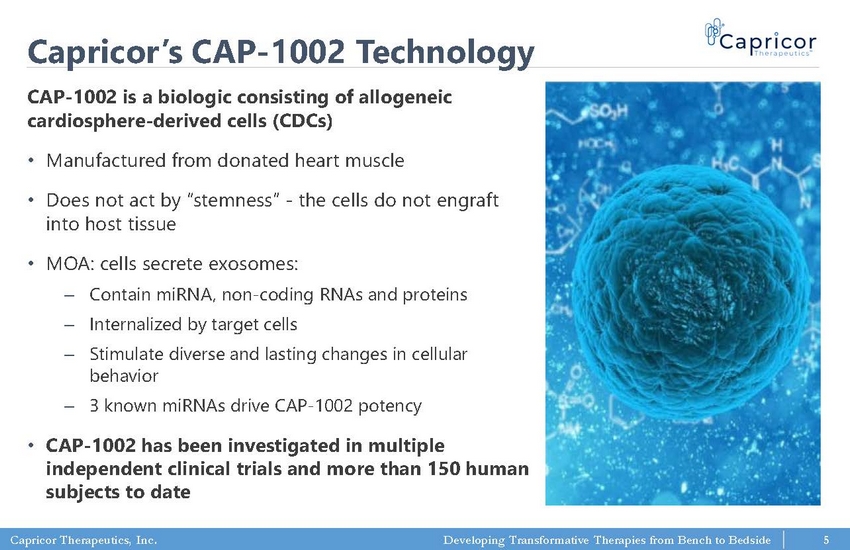
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s CAP - 1002 Technology CAP - 1002 is a biologic consisting of allogeneic cardiosphere - derived cells (CDCs) • Manufactured from donated heart muscle • Does not act by “stemness” - the cells do not engraft into host tissue • MOA: cells secrete exosomes: ‒ Contain miRNA, non - coding RNAs and proteins ‒ Internalized by target cells ‒ Stimulate diverse and lasting changes in cellular behavior ‒ 3 known miRNAs drive CAP - 1002 potency • CAP - 1002 has been investigated in multiple independent clinical trials and more than 150 human subjects to date 5

CAP - 1002 Duchenne Muscular Dystrophy Program 6
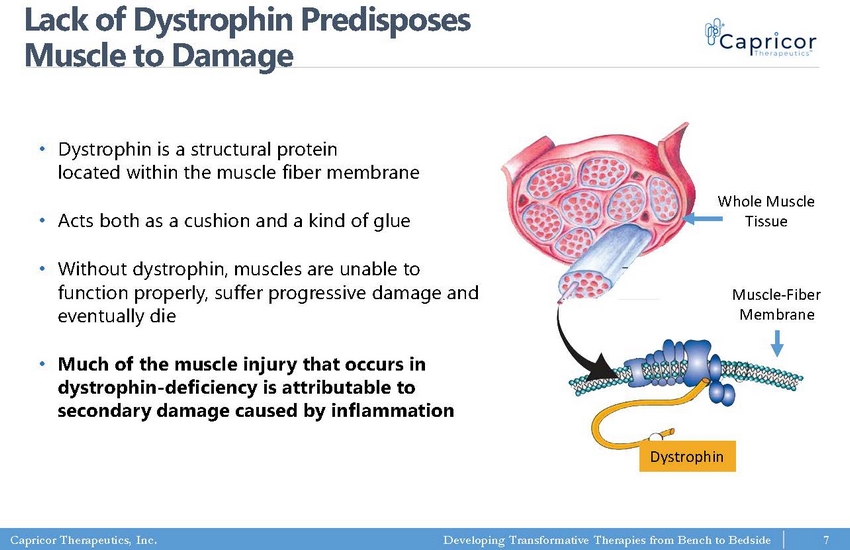
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Lack of Dystrophin Predisposes Muscle to Damage • Dystrophin is a structural protein located within the muscle fiber membrane • Acts both as a cushion and a kind of glue • Without dystrophin, muscles are unable to function properly, suffer progressive damage and eventually die • Much of the muscle injury that occurs in dystrophin - deficiency is attributable to secondary damage caused by inflammation Whole Muscle Tissue Muscle - Fiber Membrane Dystrophin 7
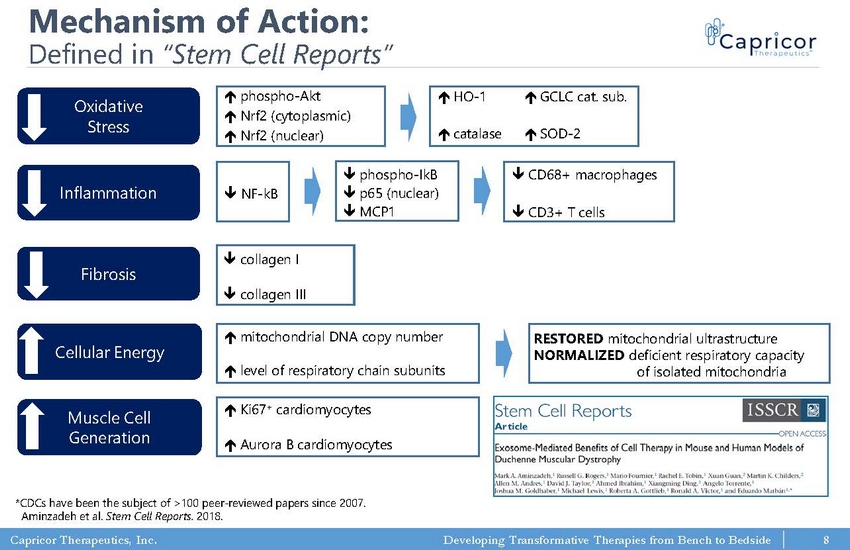
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Mechanism of Action: Defined in “Stem Cell Reports” Oxidative Stress Fibrosis Inflammation Cellular Energy Muscle Cell Generation phospho - Akt Nrf2 (cytoplasmic) Nrf2 (nuclear) HO - 1 catalase SOD - 2 GCLC cat. sub. phospho - IkB p65 (nuclear) MCP1 CD68+ macrophages CD3+ T cells collagen I collagen III m itochondrial DNA copy number level of respiratory chain subunits RESTORED mitochondrial ultrastructure NORMALIZED deficient respiratory capacity of isolated mitochondria Ki67 + cardiomyocytes Aurora B cardiomyocytes NF - kB *CDCs have been the subject of >100 peer - reviewed papers since 2007. Aminzadeh et al. Stem Cell Reports. 2018. 8
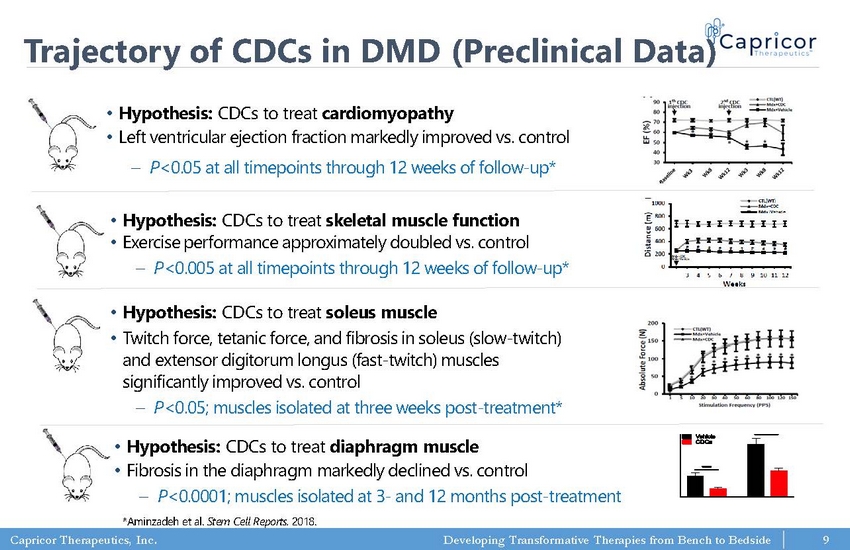
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Trajectory of CDCs in DMD (Preclinical Data) • Hypothesis: CDCs to treat cardiomyopathy • Left ventricular ejection fraction markedly improved vs. control – P <0.05 at all timepoints through 12 weeks of follow - up* • Hypothesis: CDCs to treat skeletal muscle function • Exercise performance approximately doubled vs. control – P <0.005 at all timepoints through 12 weeks of follow - up* • Hypothesis: CDCs to treat soleus muscle • Twitch force, tetanic force, and fibrosis in soleus (slow - twitch) and extensor digitorum longus (fast - twitch) muscles significantly improved vs. control – P <0.05; muscles isolated at three weeks post - treatment* *Aminzadeh et al. Stem Cell Reports. 2018. • Hypothesis: CDCs to treat diaphragm muscle • Fibrosis in the diaphragm markedly declined vs. control – P <0.0001; muscles isolated at 3 - and 12 months post - treatment 9
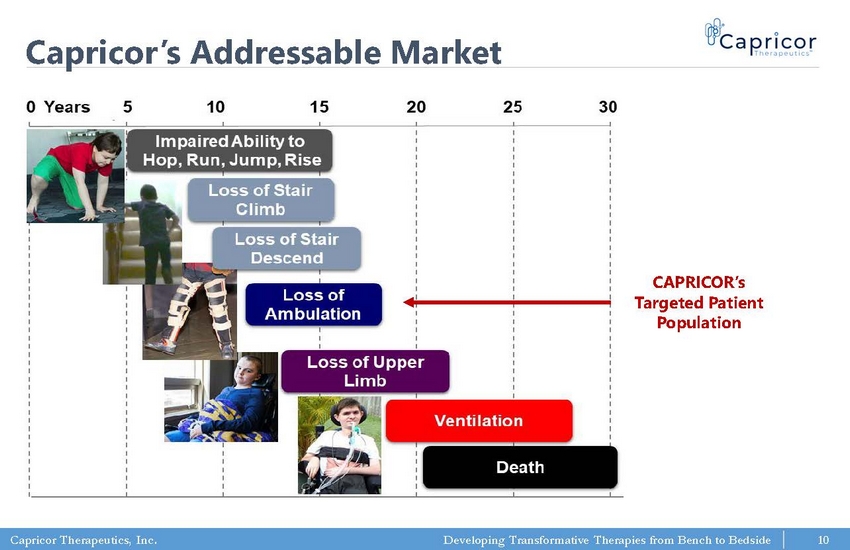
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s Addressable Market CAPRICOR’s Targeted Patient Population 10
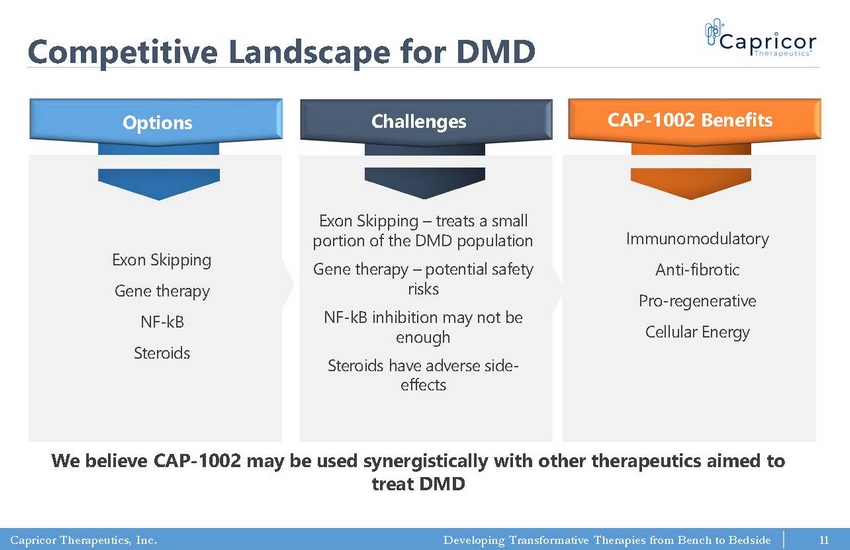
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Competitive Landscape for DMD We believe CAP - 1002 may be used synergistically with other therapeutics aimed to treat DMD Exon Skipping Gene therapy NF - kB Steroids Options Challenges CAP - 1002 Benefits Exon Skipping – treats a small portion of the DMD population Gene therapy – potential safety risks NF - kB inhibition may not be enough Steroids have adverse side - effects Immunomodulatory Anti - fibrotic Pro - regenerative Cellular Energy 11
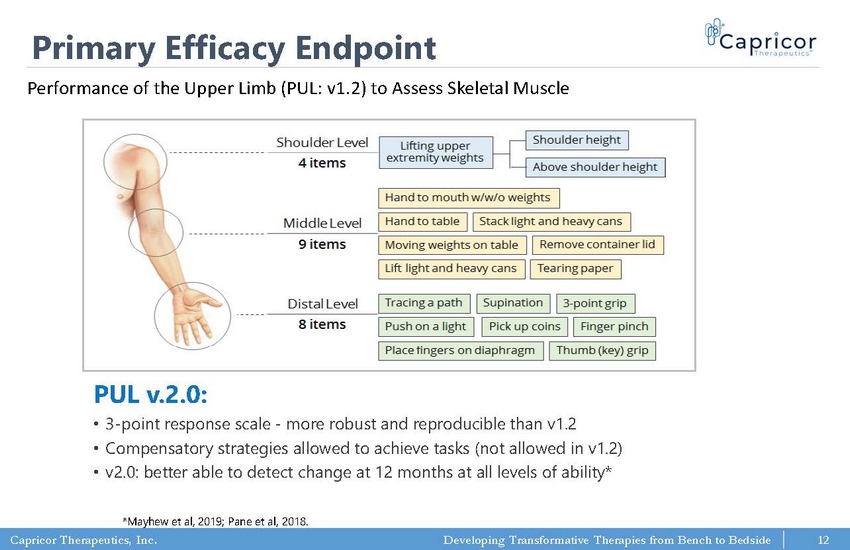
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Primary Efficacy Endpoint PUL v.2.0: • 3 - point response scale - more robust and reproducible than v1.2 • Compensatory strategies allowed to achieve tasks (not allowed in v1.2) • v2.0: better able to detect change at 12 months at all levels of ability* *Mayhew et al, 2019; Pane et al, 2018. Performance of the Upper Limb (PUL: v1.2) to Assess Skeletal Muscle 12
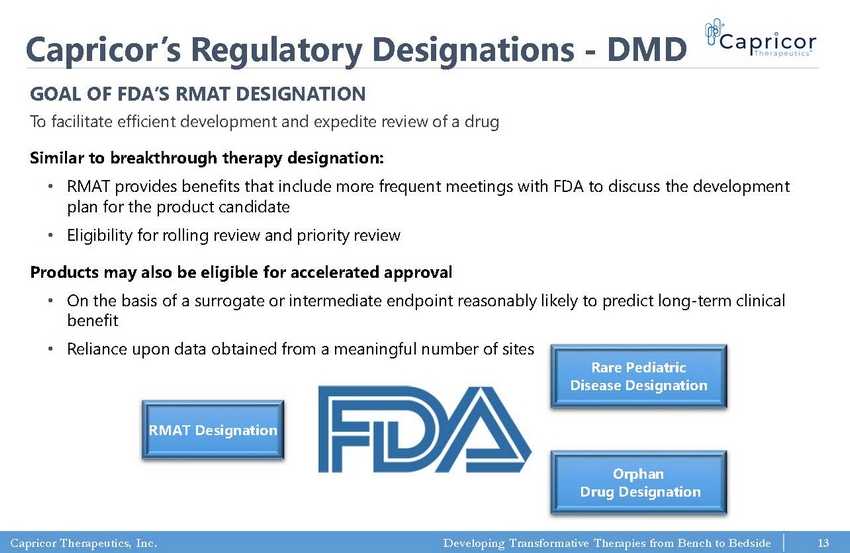
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s Regulatory Designations - DMD Similar to breakthrough therapy designation: • RMAT provides benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate • Eligibility for rolling review and priority review Products may also be eligible for accelerated approval • On the basis of a surrogate or intermediate endpoint reasonably likely to predict long - term clinical benefit • Reliance upon data obtained from a meaningful number of sites GOAL OF FDA’S RMAT DESIGNATION To facilitate efficient development and expedite review of a drug Rare Pediatric Disease Designation Orphan Drug Designation RMAT Designation 13
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - Duchenne Focused on Older DMD Patients • Phase I/II study: 25 patients, randomized and open - label • One - time, multi - vessel, intracoronary delivery of cells • HOPE population were all on stable corticosteroids • Very limited options for this patient population https://n.neurology.org/content/92/8/e866 . Study funded with the support of CIRM https://clinicaltrials.gov/ct2/show/NCT0248593 8. • Reduction in cardiac scar at 6 and 12 months measured by MRI • Improvement in cardiac function (systolic wall thickening) at 6 and 12 months • Improvements shown in PUL (mid + distal) – Best improvement shown within the first 3 months • Study published in February 2019 in Journal of Neurology RESULTS 14
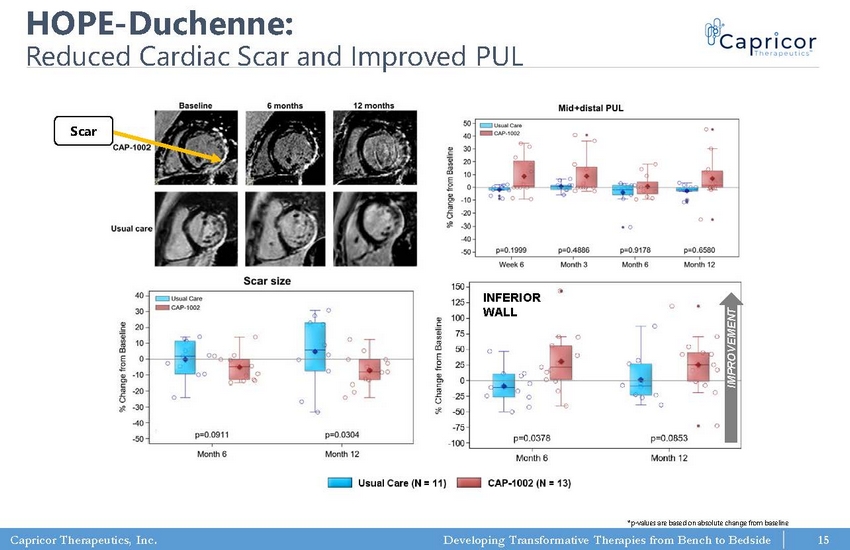
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - Duchenne: Reduced Cardiac Scar and Improved PUL R.G. Victor et al., AHA LBCT 2017; M. Taylor et al., submitted *p - values are based on absolute change from baseline INFERIOR WALL IMPROVEMENT Scar 15

HOPE - 2 Phase II Clinical Study 16
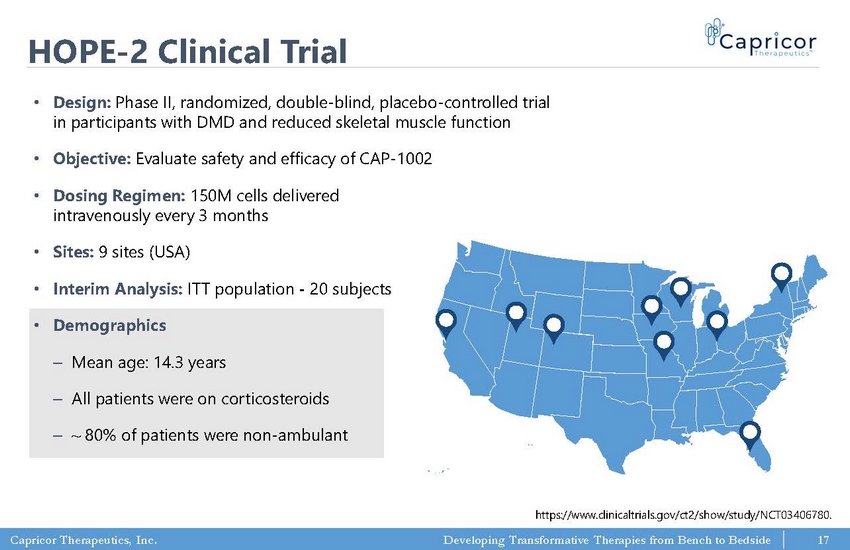
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 Clinical Trial • Design: Phase II, randomized, double - blind, placebo - controlled trial in participants with DMD and reduced skeletal muscle function • Objective: Evaluate safety and efficacy of CAP - 1002 • Dosing Regimen: 150M cells delivered intravenously every 3 months • Sites: 9 sites (USA) • Interim Analysis: ITT population - 20 subjects • Demographics ‒ Mean age: 14.3 years ‒ All patients were on corticosteroids ‒ ~ 80% of patients were non - ambulant https://www.clinicaltrials.gov/ct2/show/study/NCT03406780. 17
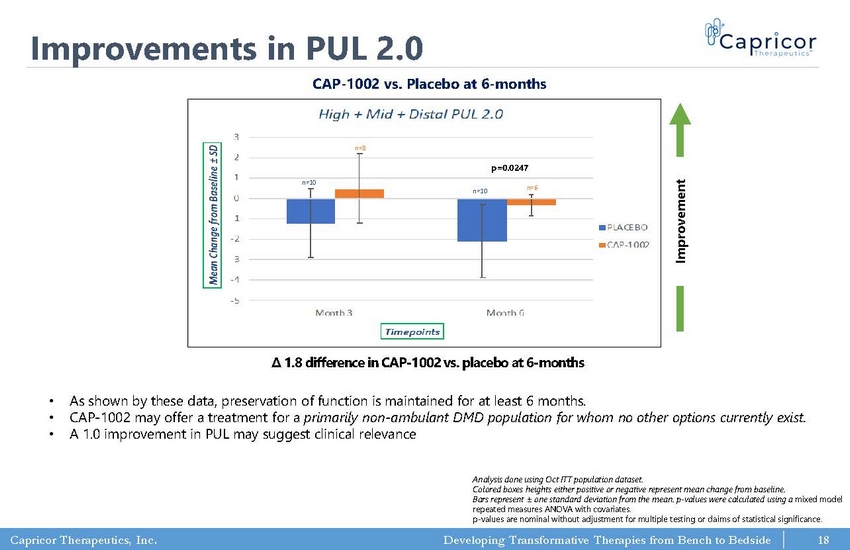
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Improvements in PUL 2.0 • As shown by these data, preservation of function is maintained for at least 6 months. • CAP - 1002 may offer a treatment for a primarily non - ambulant DMD population for whom no other options currently exist. • A 1.0 improvement in PUL may suggest clinical relevance CAP - 1002 vs. Placebo at 6 - months Improvement Analysis done using Oct ITT population dataset. Colored boxes heights either positive or negative represent mean change from baseline. Bars represent ± one standard deviation from the mean. p - values were calculated using a mixed model repeated measures ANOVA with covariates. p - values are nominal without adjustment for multiple testing or claims of statistical significance. Δ 1.8 difference in CAP - 1002 vs. placebo at 6 - months -5 -4 -3 -2 -1 0 1 2 3 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints High + Mid + Distal PUL 2.0 PLACEBO CAP-1002 n=10 n=8 n=10 n=6 p=0.0247 18
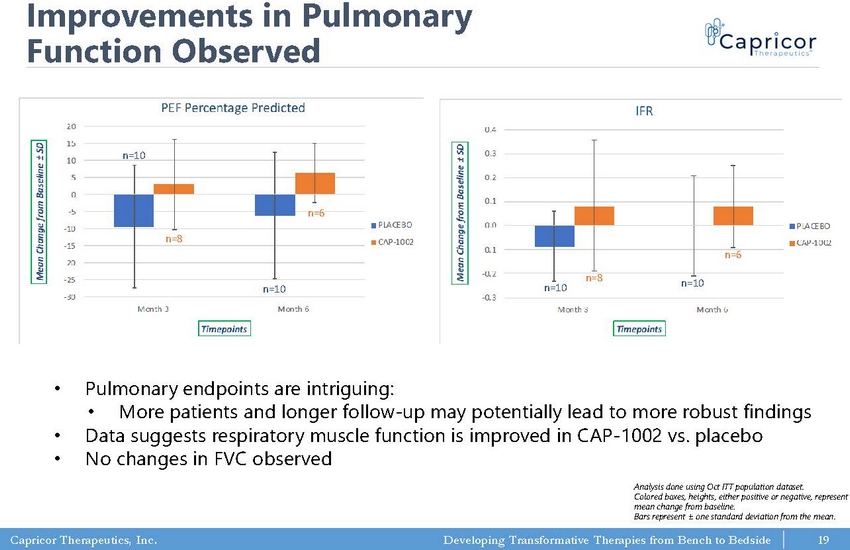
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside -30 -25 -20 -15 -10 -5 0 5 10 15 20 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints PEF Percentage Predicted PLACEBO CAP-1002 -0.3 -0.2 -0.1 0.0 0.1 0.2 0.3 0.4 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints IFR PLACEBO CAP-1002 Improvements in Pulmonary Function Observed n=10 n=8 n=10 n=6 n=10 n=8 n=10 n=6 • Pulmonary endpoints are intriguing: • More patients and longer follow - up may potentially lead to more robust findings • Data suggests respiratory muscle function is improved in CAP - 1002 vs. placebo • No changes in FVC observed Analysis done using Oct ITT population dataset. Colored boxes, heights, either positive or negative, represent mean change from baseline. Bars represent ± one standard deviation from the mean. 19
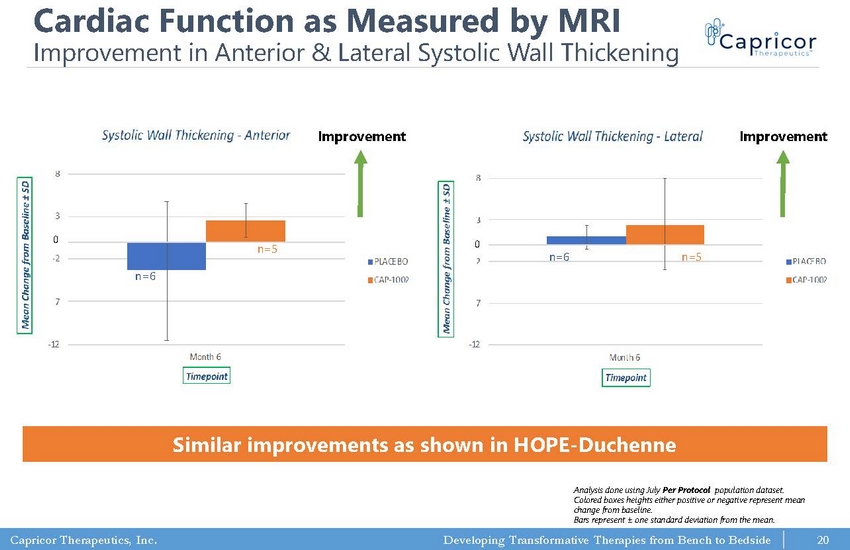
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside -12 -7 -2 3 8 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoint Systolic Wall Thickening -Anterior PLACEBO CAP-1002 -12 -7 -2 3 8 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoint Systolic Wall Thickening -Lateral PLACEBO CAP-1002 Cardiac Function as Measured by MRI Improvement in Anterior & Lateral Systolic Wall Thickening Similar improvements as shown in HOPE - Duchenne n=6 n=5 n=6 n=5 0 0 Improvement Improvement Analysis done using July Per Protocol population dataset. Colored boxes heights either positive or negative represent mean change from baseline. Bars represent ± one standard deviation from the mean. 20
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Conclusions and Future Directions CAP - 1002 for DMD • Positive outcomes from two clinical trials showing improvements in skeletal and cardiac muscle function • First placebo - controlled trial showing upper limb functional improvements in non - ambulant DMD patients • Directionally consistent improvements in function, strength, pulmonary and cardiac endpoints • Continue discussions with FDA regarding path forward • 12 - month data expected by Q2 - 2020 from HOPE - 2 • Plan to announce further updates as they become available • Continue development towards manufacturing scale - up • Pursue partnership opportunities Conclusions Moving Forward 21
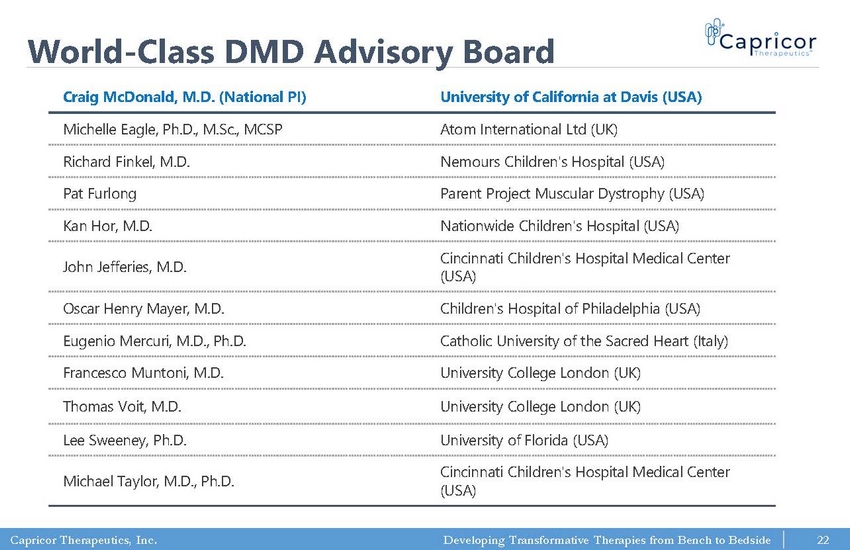
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside World - Class DMD Advisory Board Craig McDonald, M.D. (National PI) University of California at Davis (USA) Michelle Eagle, Ph.D., M.Sc., MCSP Atom International Ltd (UK) Richard Finkel, M.D. Nemours Children's Hospital (USA) Pat Furlong Parent Project Muscular Dystrophy (USA) Kan Hor, M.D. Nationwide Children's Hospital (USA) John Jefferies, M.D. Cincinnati Children's Hospital Medical Center (USA) Oscar Henry Mayer, M.D. Children's Hospital of Philadelphia (USA) Eugenio Mercuri, M.D., Ph.D. Catholic University of the Sacred Heart (Italy) Francesco Muntoni, M.D. University College London (UK) Thomas Voit , M.D. University College London (UK) Lee Sweeney, Ph.D. University of Florida (USA) Michael Taylor, M.D., Ph.D. Cincinnati Children's Hospital Medical Center (USA) 22

Exosomes Technology 23

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside From Discovery to Platform Development 24 Publications covering our technology have been published by us or our collaborators in multiple peer - reviewed journals.
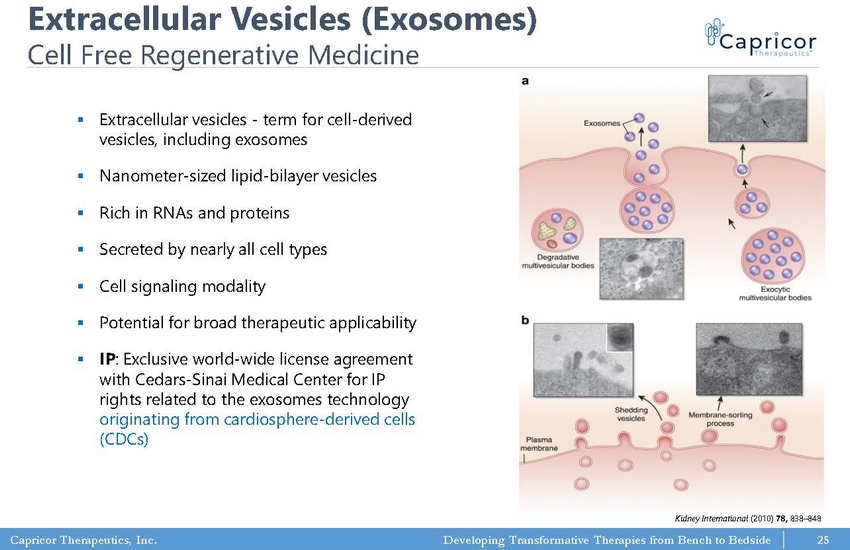
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Extracellular Vesicles (Exosomes) Cell Free Regenerative Medicine ▪ Extracellular vesicles - term for cell - derived vesicles, including exosomes ▪ Nanometer - sized lipid - bilayer vesicles ▪ Rich in RNAs and proteins ▪ Secreted by nearly all cell types ▪ Cell signaling modality ▪ Potential for broad therapeutic applicability ▪ IP : Exclusive world - wide license agreement with Cedars - Sinai Medical Center for IP rights related to the exosomes technology originating from cardiosphere - derived cells (CDCs) Kidney International (2010) 78, 838 – 848 25
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside The Field of Exosomes: Increasing Market Opportunity 26 Problems Possible Solutions Large Addressable Market The global market for exosome diagnostic and therapeutics is expected to reach revenue of $580 million by 2027 1 High Growth 27.7% CAGR of the global exosome diagnostic and therapeutic market through 2019 to 2027 1 Increased Financing Activities Over $300M raised to date in several private companies to date 2 Increased Strategic Transactions M&A activity continuing to increase in delivery, oncology and manufacturing with therapeutics underrepresented in deal flow 1 https://www.reportlinker.com/p05775295/GLOBAL - EXOSOME - DIAGNOSTIC - AND - THERAPEUTIC - MARKET - FORECAST.html?utm_source=PRN 2 Based on public data online sources
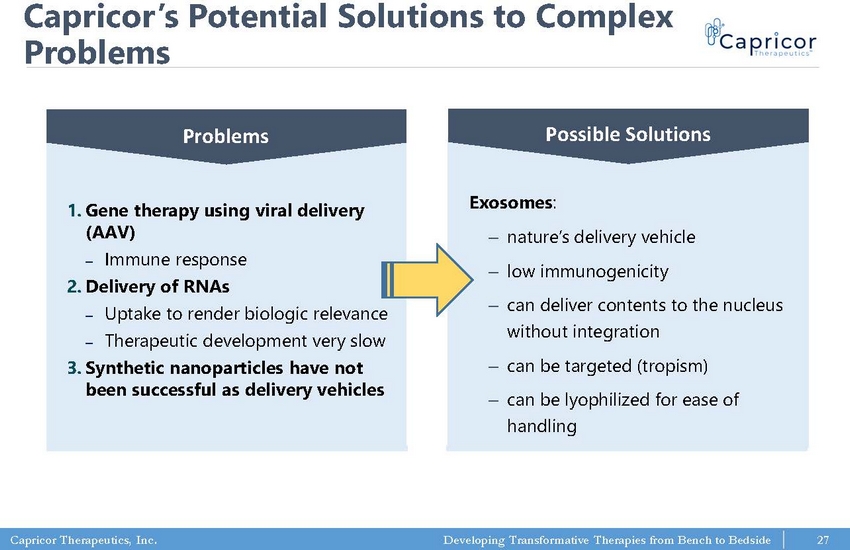
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s Potential Solutions to Complex Problems 1. Gene therapy using viral delivery (AAV) ‒ Immune response 2. Delivery of RNAs ‒ Uptake to render biologic relevance ‒ Therapeutic development very slow 3. Synthetic nanoparticles have not been successful as delivery vehicles 27 Exosomes : ‒ nature’s delivery vehicle ‒ low immunogenicity ‒ can deliver contents to the nucleus without integration ‒ can be targeted (tropism) ‒ can be lyophilized for ease of handling Problems Possible Solutions
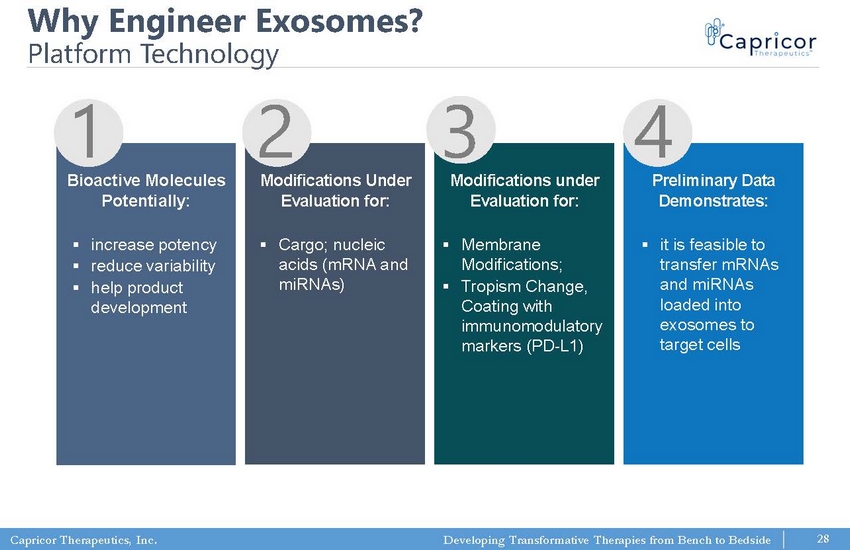
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Why Engineer Exosomes? Platform Technology 28 ▪ increase potency ▪ reduce variability ▪ help product development ▪ Cargo; nucleic acids (mRNA and miRNAs) ▪ Membrane Modifications; ▪ Tropism Change, Coating with immunomodulatory markers (PD - L1) ▪ it is feasible to transfer mRNAs and miRNAs loaded into exosomes to target cells 1 2 3 4 Preliminary Data Demonstrates: Modifications under Evaluation for: Modifications Under Evaluation for: Bioactive Molecules Potentially:
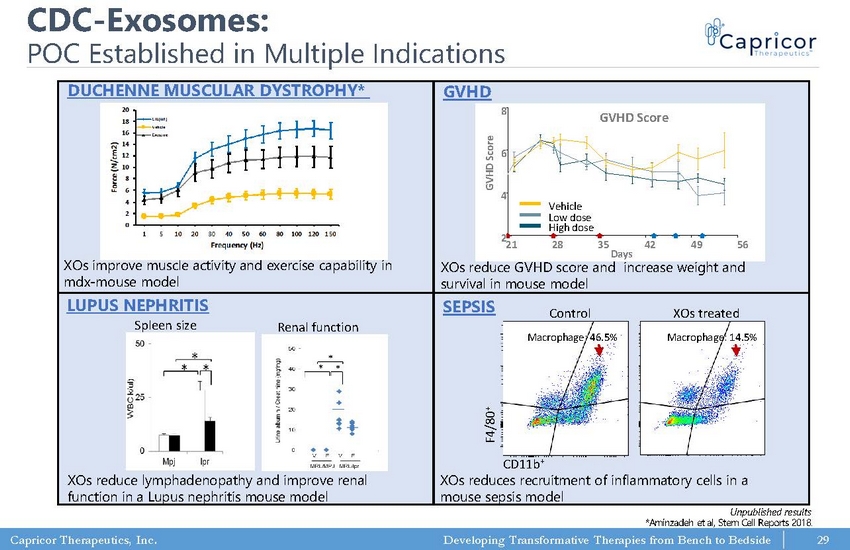
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside CDC - Exosomes: POC Established in Multiple Indications LUPUS NEPHRITIS SEPSIS F4/80 + CD11b + Macrophage: 46.5% Macrophage: 14.5% Renal function Spleen size XOs reduce lymphadenopathy and improve renal function in a Lupus nephritis mouse model Control XOs treated XOs reduces recruitment of inflammatory cells in a mouse sepsis model Unpublished results *Aminzadeh et al, Stem Cell Reports 2018. 29 DUCHENNE MUSCULAR DYSTROPHY* XOs improve muscle activity and exercise capability in mdx - mouse model 0 2 4 6 8 10 12 14 16 18 20 1 5 10 20 30 40 50 60 80 100 120 150 F o r c e ( N / c m 2 ) Frequency (Hz) CTL(WT) Vehicle Exosome GVHD 21 28 35 42 49 56 2 4 6 8 Day G V H D S c o r e Vehicle Low dose High Dose 21 28 35 42 49 56 Days 8 6 4 2 GVHD Score GVHD Score Vehicle Low dose High dose XOs reduce GVHD score and increase weight and survival in mouse model
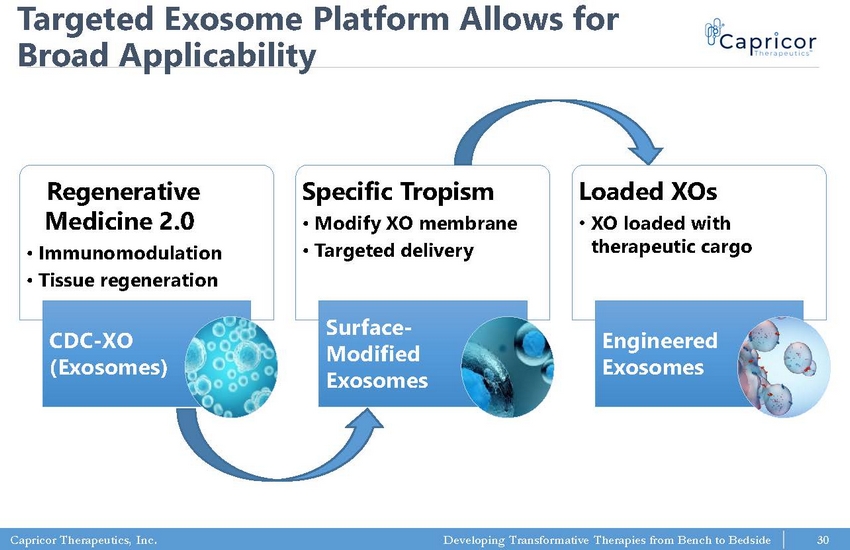
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Targeted Exosome Platform Allows for Broad Applicability Regenerative Medicine 2.0 • Immunomodulation • Tissue regeneration CDC - XO (Exosomes) Specific Tropism • Modify XO membrane • Targeted delivery Surface - Modified Exosomes Loaded XOs • XO loaded with therapeutic cargo Engineered Exosomes 30

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Recent and Targeted Catalysts in 2020 31 CAP - 1002 in Duchenne Muscular Dystrophy x Feb. 2019: HOPE - Duchenne (Phase I/II) study published in Journal of Neurology x Oct. 2019 : Reported positive six - month results of HOPE - 2 trial at World Muscle Society International Conference x Oct. 2019: Met with FDA to discuss DMD program x Dec. 2019: Completed $5.1M offering (priced at - market) □ April 2020: Plan to host KOL call on cardiac complications in DMD □ Q2 - 2020: Plan to announce HOPE - 2 final 12 - month results □ Mid - 2020 : Plan to m eet with FDA to discuss CAP - 1002 in DMD □ 2H - 2020: Plan to present HOPE - 2 final results at medical conference □ 2020: Continue to pursue partnership opportunities for DMD program Exosomes Platform Technology □ April - 2020 : Plan to submit IND for DMD □ 1H - 2020: Planning key hires in R&D □ 2H - 2020: Aim to publish data from exosomes technology □ 2020: Continue to advance platform opportunities

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Senior Leadership Team Linda Marbán, Ph.D. Chief Executive Officer, Co - founder and Director ▪ Dr. Marbán has over 25 years of experience in the biotechnology industry ▪ Been with Capricor since 2005 and CEO since 2010. ▪ Previous experience includes Excigen, Inc. where she was responsible for business development and operations. ▪ Dr. Marbán began her career in academic science at the Cleveland Clinic Foundation working on the biophysical properties of cardiac muscle and continued to a postdoctoral fellowship at Johns Hopkins University. ▪ Dr. Marbán earned a Ph.D. from Case Western Reserve University in cardiac physiology. Karen Krasney, J.D. Executive Vice President & General Counsel ▪ Ms. Krasney’s has over 40 years of experience in domestic and international corporate and business law, as well as litigation. ▪ Ms. Krasney served as legal counsel of Biosensors International Group Ltd., a multinational medical device company. ▪ Ms. Krasney received her Bachelor of Arts degree from the University of California, Los Angeles and her Juris Doctorate from the University of Southern California. Tariq Warsi, Ph.D. Vice President of Research and Development ▪ Dr. Warsi has over 20 years of experience in cell and molecular biology. ▪ Dr. Warsi held positions at Amgen® where he performed research in cell and gene therapy, immunotherapy and large protein biologics contributing to the delivery of analytical methods, process optimization, authoring INDs and leading programs from R&D through early phase clinical studies. ▪ Dr. Warsi earned his Bachelor of Science degree in Biological Sciences from the University of California, Riverside. ▪ He conducted his postdoctoral research at Harvard Medical School, where he studied the contributions of protein structure, function and degradation. AJ Bergmann, M . B . A . Chief Financial Officer ▪ Mr. Bergmann has worked in the finance industry for over a decade. ▪ Mr. Bergmann joined Capricor in 2011 and coordinated the Company’s reverse merger, uplisting to NASDAQ and financings yielding over $50 million to date. ▪ Mr. Bergmann graduated from Providence College and has a M.B.A. from the University of Southern California’s Marshall School of Business. Siegfried Rogy , Ph.D. Vice President of Clinical Operations ▪ Dr. Rogy has over 25 years of clinical operations and development experience at companies including Baxter Bioscience, The Medicines Company and Maxim Pharmaceuticals. ▪ Dr. Rogy held positions at two start - up biotech companies including Novalar where he successfully directed a Phase I - III clinical program leading to the marketing authorization of OraVerse ®, a local anesthesia reversal agent. ▪ Dr. Rogy earned his Bachelor of Science and Ph.D. in Biology from the Karl - Franzens - University, Graz, Austria. 32