
Exhibit 99.1

NASDAQ: CAPR A Translational Medicine Company www.capricor.com Investor Presentation October 2016

2 This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc . (Capricor) as well as assumptions made by and information currently available to Capricor . All statements other than statements of historical fact included in this presentation are forward - looking statements, including but not limited to statements identified by the words “anticipates,” “believes,” “estimates,” and “expects” and similar expressions . Such forward - looking statements also include any expectation of or dates for commencement of clinical trials, IND filings, similar plans or projections and other matters that do not relate strictly to historical facts . These statements reflect Capricor’s current views with respect to future events, based on what we believe are reasonable assumptions ; however, the statements are subject to a number of risks, uncertainties and assumptions . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business are set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2015 , as filed with the Securities and Exchange Commission on March 30 , 2016 , in its Registration Statement on Form S - 3 , as filed with the Securities and Exchange Commission on September 28 , 2015 and in its Quarterly Report on Form 10 - Q for the period ended June 30 , 2016 as filed with the Securities and Exchange Commission on August 15 , 2016 . Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those in the forward - looking statements . Further, Capricor’s management does not intend to update these forward - looking statements and information after the date of this presentation . Forward - Looking Statements
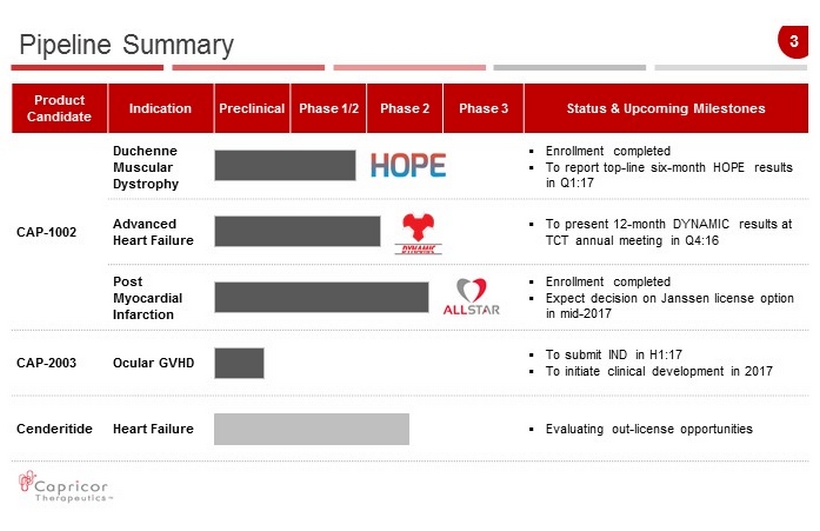
3 Pipeline Summary Product Candidate Indication Preclinical Phase 1/2 Phase 2 Phase 3 Status & Upcoming Milestones CAP - 1002 Duchenne Muscular Dystrophy ▪ Enrollment completed ▪ To report to p - line six - month HOPE results in Q1:17 Advanced Heart Failure ▪ To present 12 - month DYNAMIC results at TCT annual meeting in Q4:16 Post Myocardial Infarction ▪ Enrollment completed ▪ Expect decision on Janssen license option in mid - 2017 CAP - 2003 Ocular GVHD ▪ To submit IND in H1:17 ▪ To in itiate clinical development in 2017 Cenderitide Heart Failure ▪ Evaluating o ut - licens e opportunities
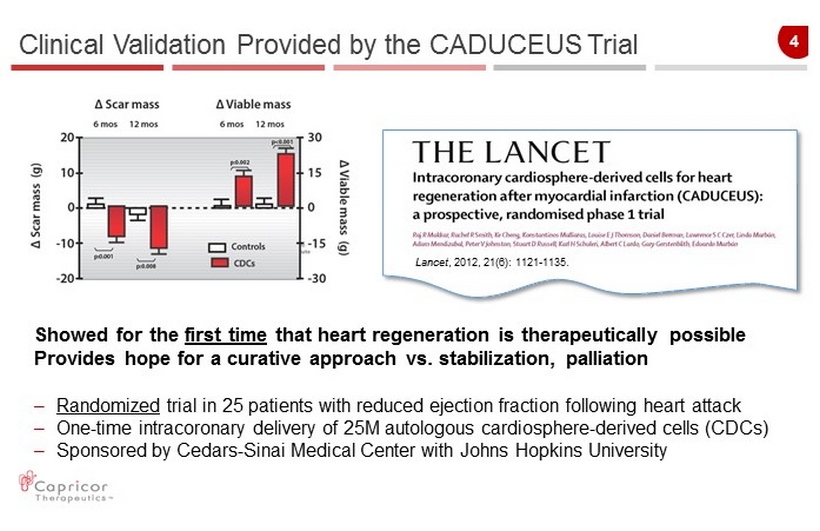
4 Clinical Validation Provided by the CADUCEUS Trial Lancet , 2012, 21(6): 1121 - 1135. Showed for the first time that heart regeneration is therapeutically possible Provides hope for a curative approach vs. stabilization, palliation – Randomized trial in 25 patients with reduced ejection fraction following heart attack – One - time intracoronary delivery of 25M autologous cardiosphere - derived cells (CDCs) – Sponsored by Cedars - Sinai Medical Center with Johns Hopkins University
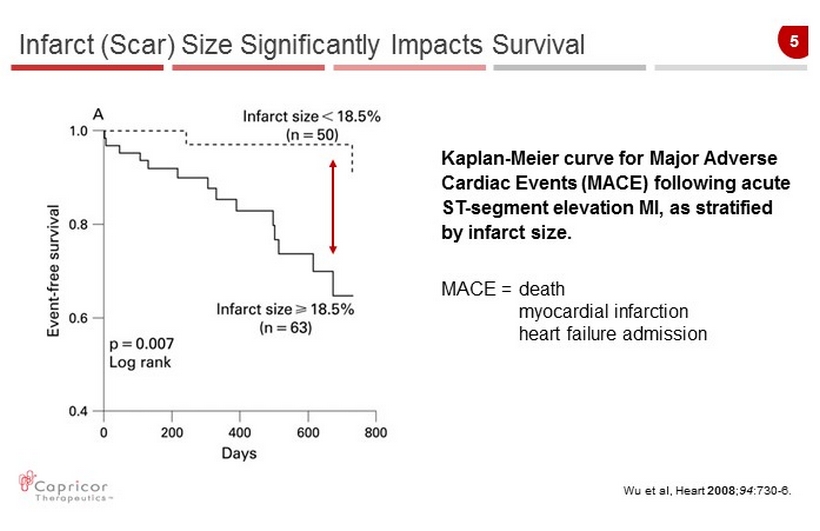
5 Infarct (Scar) Size Significantly Impacts Survival Wu et al, Heart 2008 ; 94 :730 - 6. Kaplan - Meier curve for Major Adverse Cardiac Events (MACE) following acute ST - segment elevation MI, as stratified by infarct size. MACE = death myocardial infarction heart failure admission
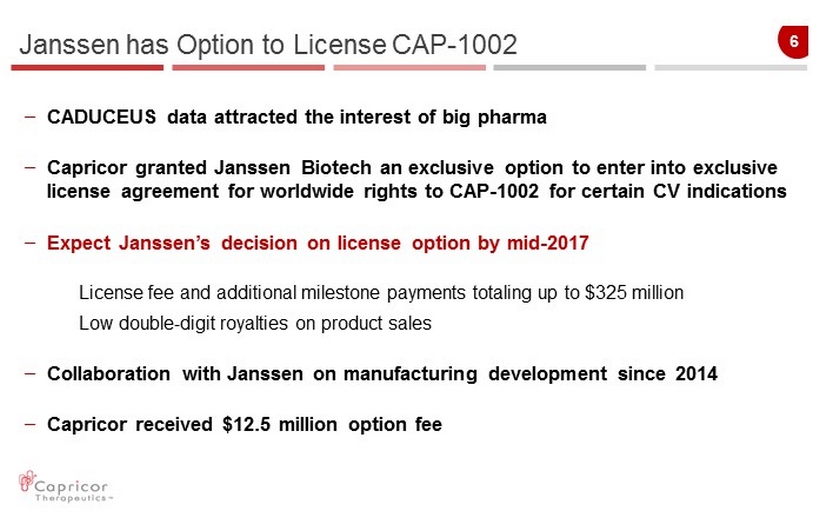
6 – CADUCEUS data attracted the interest of big pharma – Capricor granted Janssen Biotech an exclusive option to enter into exclusive license agreement for worldwide rights to CAP - 1002 for certain CV indications – Expect Janssen’s decision on license option by mid - 2017 License fee and additional milestone payments totaling up to $325 million Low double - digit royalties on product sales – Collaboration with Janssen on manufacturing development since 2014 – Capricor received $12.5 million option fee Janssen has Option to License CAP - 1002

7 Heart Cells for Heart Disease Capricor’s core technology is the cardiosphere - derived cell (CDC): – Does not act by ‘ stemness ’ – does not engraft into myocardium – Exerts favorable and durable effects on target cells via exosome signalling – cardiomyogenic , anti - apoptotic, angiogenic , anti - fibrotic CAP - 1002 (allogeneic CDCs) is an “off the shelf” product – One donor heart can provide thousands of doses of CAP - 1002 – Product is packaged in Cryostor ™, has 3 - year frozen shelf life – Clinical material currently produced at Capricor facility via proprietary process – Commercial - scale process being developed in collaboration with Janssen Record of immunological safety per cumulative clinical experience IP licensed from Cedars - Sinai Medical Center, Johns Hopkins, U. of Rome
8 Capricor – Portfolio at a Glance CAP - 1002 CAP - 2003 Natriuretic Peptides Adult Cardiac Conditions P ROGRAM Phase II
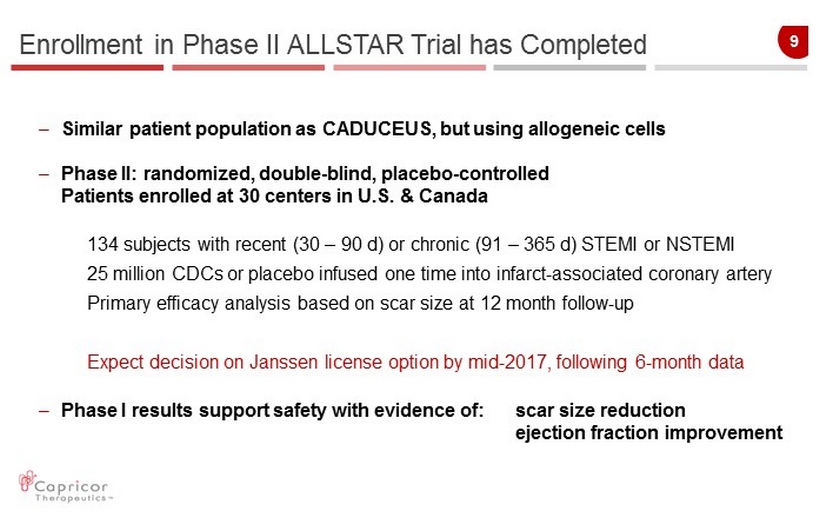
9 Enrollment in Phase II ALLSTAR Trial has Completed – Similar patient population as CADUCEUS, but using allogeneic cells – Phase II: randomized , double - blind, placebo - controlled Patients enrolled at 30 centers in U.S. & Canada 134 subjects with recent (30 – 90 d) or chronic (91 – 365 d) STEMI or NSTEMI 25 million CDCs or placebo infused one time into infarct - associated coronary artery Primary efficacy analysis based on scar size at 12 month follow - up Expect decision on Janssen license option by mid - 2017, following 6 - month data – Phase I results support safety with evidence of: scar size reduction ejection fraction improvement
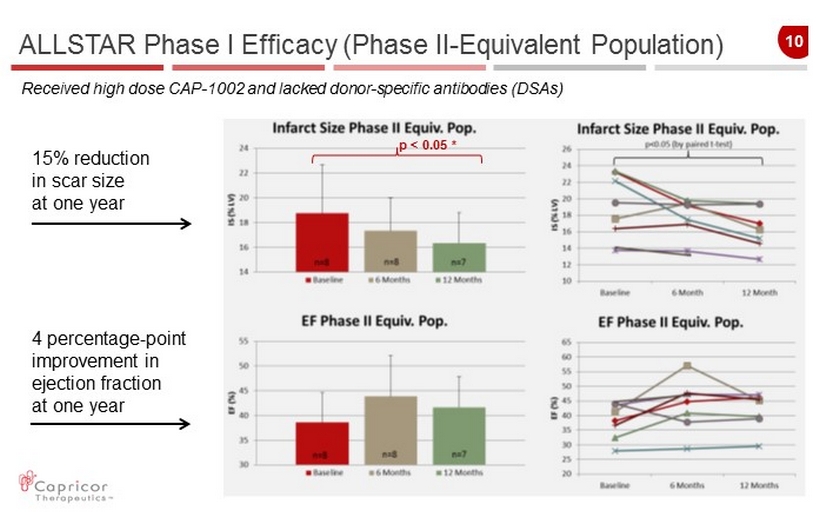
10 p < 0.05 * ALLSTAR Phase I Efficacy (Phase II - Equivalent Population) 15 % reduction in scar size at one year 4 percentage - point improvement in ejection fraction at one year Received high dose CAP - 1002 and lacked donor - specific antibodies ( DSAs)
11 DYNAMIC Clinical Trial of CAP - 1002 in Advanced HF – Phase IIa , open - label , dose - escalation clinical trial in heart failure patients C onducted at Cedars - Sinai Medical Center – Enrollment was open to subjects with dilated cardiomyopathy NYHA Class III or ambulatory Class IV I schemic or non - ischemic origin – Baseline LV ejection fraction ≤ 35% – One - time triple coronary infusion at one of four doses 37.5 , 50, 62.5, or 75 million cells – Six and twelve - month follow - up
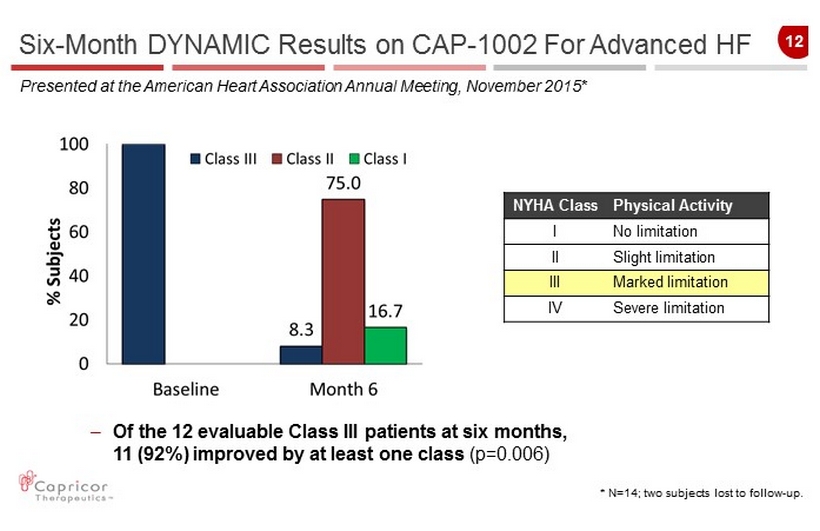
12 NYHA Class Physical Activity I No limitation II Slight limitation III Marked limitation IV Severe limitation * N=14 ; two subjects lost to follow - up. Six - Month DYNAMIC Results on CAP - 1002 For Advanced HF Presented at the American Heart Association Annual Meeting, November 2015* – Of the 12 evaluable Class III patients at six months, 11 (92%) improved by at least one class (p=0.006)
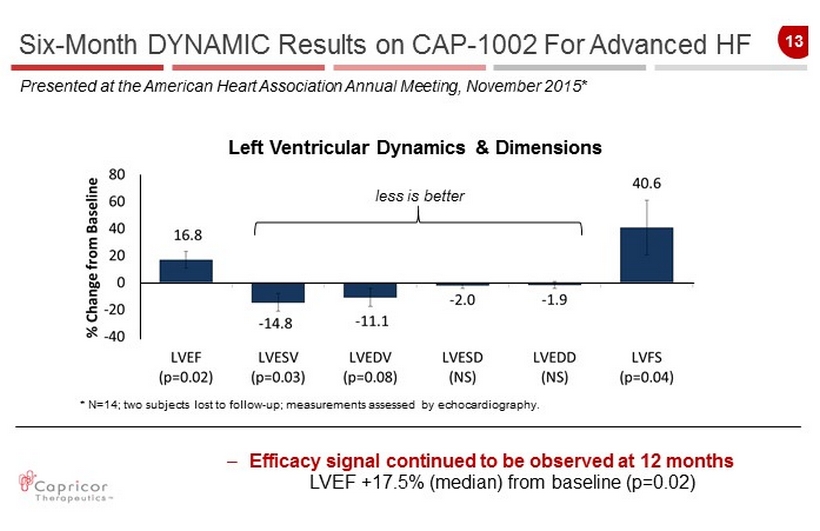
13 Left Ventricular Dynamics & Dimensions less is better * N=14; two subjects lost to follow - up; measurements assessed by echocardiography . Six - Month DYNAMIC Results on CAP - 1002 For Advanced HF Presented at the American Heart Association Annual Meeting, November 2015* – Efficacy signal continued to be observed at 12 months LVEF +17.5% (median) from baseline (p=0.02)
14 Capricor – Portfolio at a Glance CAP - 1002 CAP - 2003 Natriuretic Peptides Duchenne Muscular Dystrophy P ROGRAM Phase I / II
15 Cardiomyopathy is #1 Cause of Death in DMD – DMD results from mutation in dystrophin gene – 1 per 3,600 male births – Lack of functional dystrophin in heart leads to: inflammation cardiomyocyte death progressive cardiac fibrosis – Hearts become dilated and non - compliant, and eventually fail – No approved therapies for heart disease associated with DMD
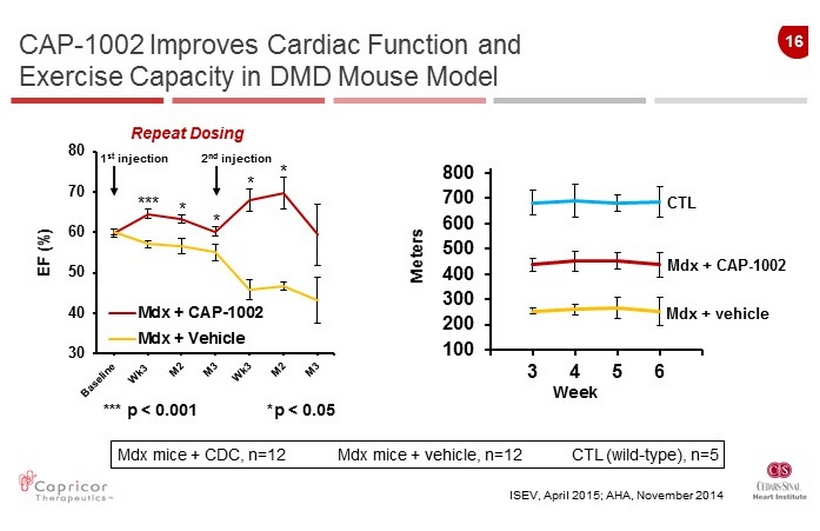
16 30 40 50 60 70 80 Mdx + CAP-1002 Mdx + Vehicle *** p < 0.001 * p < 0.05 100 200 300 400 500 600 700 800 3 4 5 6 CTL Mdx + CAP - 1002 Mdx + vehicle Week *** 1 st injection EF (%) Repeat Dosing Meters 2 nd injection Mdx mice + CDC, n=12 Mdx mice + vehicle, n=12 CTL (wild - type), n=5 CAP - 1002 Improves Cardiac Function and Exercise Capacity in DMD Mouse Model ISEV, April 2015; AHA , November 2014 * * * *
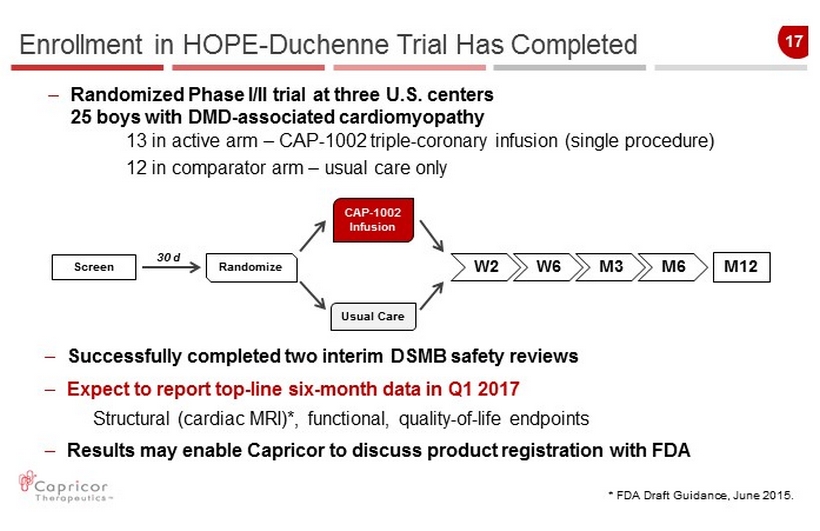
17 – Successfully completed two interim DSMB safety reviews – Expect to report top - line six - month data in Q1 2017 Structural (cardiac MRI)*, functional, quality - of - life endpoints – Results may enable Capricor to discuss product registration with FDA Randomize Usual Care M12 W2 W6 M3 M6 30 d Enrollment in HOPE - Duchenne Trial Has Completed Screen * FDA Draft Guidance, June 2015. CAP - 1002 Infusion – Randomized Phase I/II trial at three U.S. centers 25 boys with DMD - associated cardiomyopathy 13 in active arm – CAP - 1002 triple - coronary infusion (single procedure) 12 in comparator arm – usual care only
18 Capricor – Portfolio at a Glance Diseases of Inflammation and Fibrosis CAP - 1002 CAP - 2003 Natriuretic Peptides P ROGRAM Preclinical
19 Exosomes: Cell Free Regenerative Medicine Platform Camussi et al, Kidney Int 2010 ; 78 :838 – 848. – CDC Exosomes are virus - sized vesicles that can reduce inflammation and regenerate damaged tissue – CAP - 2003 (CDC Exosomes ) may be used in applications for which CDCs may not be well - suited – Exclusive WW license from Cedars - Sinai Medical Center to CDC E xosomes technology – Ocular graft - vs - host disease ( oGVHD ) i s first clinical indication for CAP - 2003
20 Ocular GVHD – First Clinical Development Opportunity – Graft - vs - host disease (GVHD) A common complication of bone marrow transplantation (BMT) Chronic rejection of new cells – Ocular GVHD is a common, debilitating form of chronic GVHD and is an unmet medical need Burning, irritation, photophobia, pain, dryness No approved therapies Orphan disease: 8,500 BMT are performed in the U.S. annually 40% of BMT recipients suffer from oGVHD – Capricor attended a pre - IND meeting with FDA in July 2016 Expect to submit IND application for CAP - 2003 in oGVHD in H1:17 Alkali burn model sufficient for preclinical POC http:// bloodcell.transplant.hrsa.gov/about/general_faqs/index.html Shikari et al, Surv Ophthalmol . 2013 May - Jun;58(3): 233 - 51. ~3,400 new cases p er year
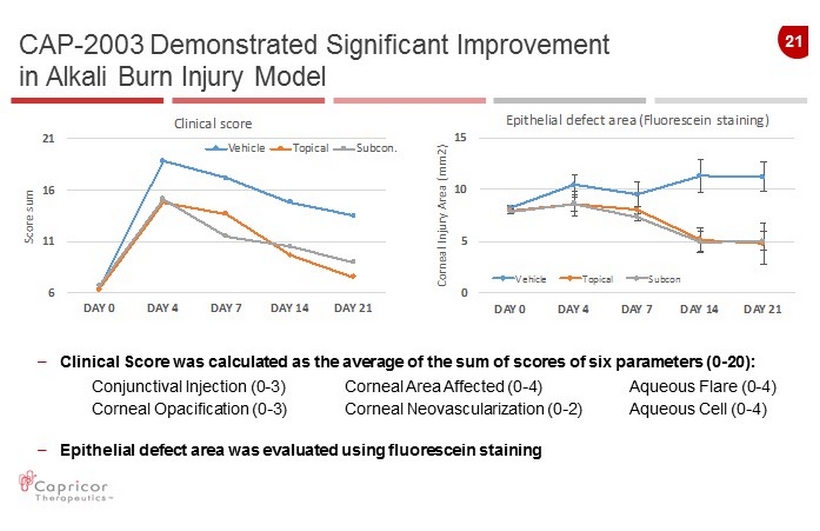
21 CAP - 2003 Demonstrated Significant Improvement in Alkali Burn Injury Model 6 11 16 21 DAY 0 DAY 4 DAY 7 DAY 14 DAY 21 S c o r e s u m Clinical score Vehicle Topical Subcon. 0 5 10 15 DAY 0 DAY 4 DAY 7 DAY 14 DAY 21 C o r n e a l I n j u r y A r e a ( m m 2 ) Epithelial defect area (Fluorescein staining) Vehicle Topical Subcon – Clinical Score was calculated as the average of the sum of scores of six parameters ( 0 - 20): Conjunctival Injection (0 - 3) Corneal Area Affected (0 - 4) Aqueous Flare (0 - 4) Corneal Opacification (0 - 3) Corneal Neovascularization (0 - 2) Aqueous Cell (0 - 4) – Epithelial defect area was evaluated using fluorescein staining
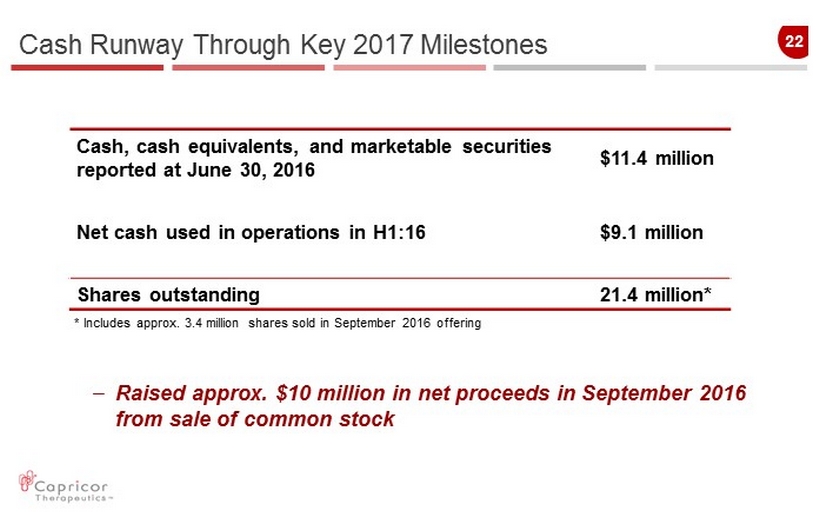
22 Cash, cash equivalents, and marketable securities reported at June 30, 2016 $11.4 million Net cash used in operations in H1:16 $9.1 million Shares outstanding 21.4 million* Cash Runway Through Key 2017 Milestones – Raised approx. $10 million in net proceeds in September 2016 from sale of common stock * Includes approx. 3.4 million shares sold in September 2016 offering

23 Recent and Upcoming Milestones CAP - 1002 in Duchenne Heart Disease x Announced completion of HOPE enrollment in September 2016 □ Expect to report six - month top - line results of HOPE in Q1:17 CAP - 1002 in Adult Heart Disease x Announced completion of ALLSTAR enrollment in October 2016 □ To present 12 - month DYNAMIC data at TCT in Q4:16 □ Expect Janssen decision on license option by mid - 2017 CAP - 2003 x Demonstrated evidence of activity in several pre - clinical disease models □ Expect to submit IND application for oGVHD in H1:17

NASDAQ: CAPR A Translational Medicine Company www.capricor.com 8840 Wilshire Boulevard – 2 nd floor Beverly Hills, CA 90211 (310) 358 - 3200