
Exhibit 99.1

NASDAQ: CAPR April 25, 2017 a translational m edicine c ompany Top - Line Six - Month Results of the HOPE - Duchenne Trial Conference Call to Discuss

2 This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc . (Capricor) as well as assumptions made by and information currently available to Capricor . All statements other than statements of historical fact included in this presentation are forward - looking statements, including but not limited to statements identified by the words “anticipates,” “believes,” “estimates,” and “expects” and similar expressions . Such forward - looking statements also include any expectation of or dates for commencement of clinical trials, IND filings, similar plans or projections and other matters that do not relate strictly to historical facts . These statements reflect Capricor’s current views with respect to future events, based on what we believe are reasonable assumptions ; however, the statements are subject to a number of risks, uncertainties and assumptions . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2016 , as filed with the Securities and Exchange Commission on March 16 , 2017 , and in its Registration Statement on Form S - 3 , as filed with the Securities and Exchange Commission on September 28 , 2015 , together with prospectus supplements thereto . Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those in the forward - looking statements . Further, Capricor’s management does not intend to update these forward - looking statements and information after the date of this presentation . Forward - Looking Statements
3 Positive Top - Line Six - Month Safety and Exploratory Efficacy Data — Statistically - significant i mprovements were observed in exploratory efficacy measures of cardiac and upper limb function in patients who received CAP - 1002 — Clinical results recapitulate pre - clinical data in cardiac and skeletal muscle — CAP - 1002 was generally safe and well - tolerated — Capricor has submitted a meeting request to the FDA to discuss potential product registration strategies for CAP - 1002 in the DMD indication — Plan to submit HOPE data to FDA in support of potential Breakthrough Therapy and Regenerative Medicine Advanced Technology (RMAT) designations Capricor to Advance Clinical Development of CAP - 1002 as a Treatment for the Skeletal and Cardiac Manifestations of DMD

4 Duchenne Muscular Dystrophy (DMD) and CAP - 1002 — DMD is a genetic disorder characterized by progressive and debilitating muscle weakness ▪ Results from a lack of functional dystrophin ▪ Affects cardiac muscle and all skeletal muscle ▪ Muscle cell death due to increased fragility ▪ Capacity of muscle to regenerate diminishes over time, then is lost ▪ Replacement of muscle with scar and fat — In studies in Mdx mice, CDCs improve: ▪ Cardiac performance and scar ▪ Running speed and distance ▪ Contractile force of isolated skeletal muscles
5 About CAP - 1002 (allogenic cardiosphere - derived cells) — CAP - 1002 is a cardiac progenitor cell therapeutic candidate ▪ CDCs have been shown to lead to scar regression and generate functional cardiac muscle — Mechanisms of action of CAP - 1002: ▪ A nti - inflammatory, anti - fibrotic , and anti - apoptotic ▪ Affect mitochondrial structure, oxidative function and cell morphology ▪ Pre - print manuscript available at http :// biorxiv.org/content/early/2017/04/20/128900
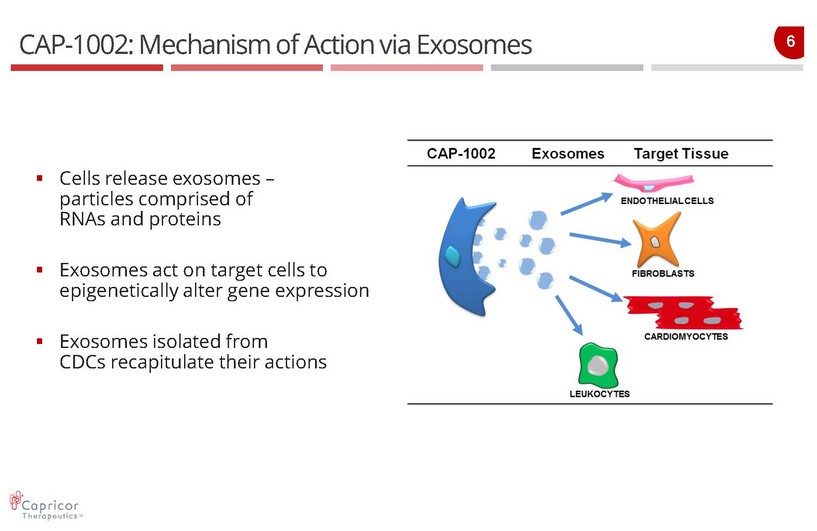
6 CAP - 1002: Mechanism of Action via Exosomes ▪ Cells release exosomes – particles comprised of RNAs and proteins ▪ Exosomes act on target cells to epigenetically alter gene expression ▪ Exosomes isolated from CDCs recapitulate their actions
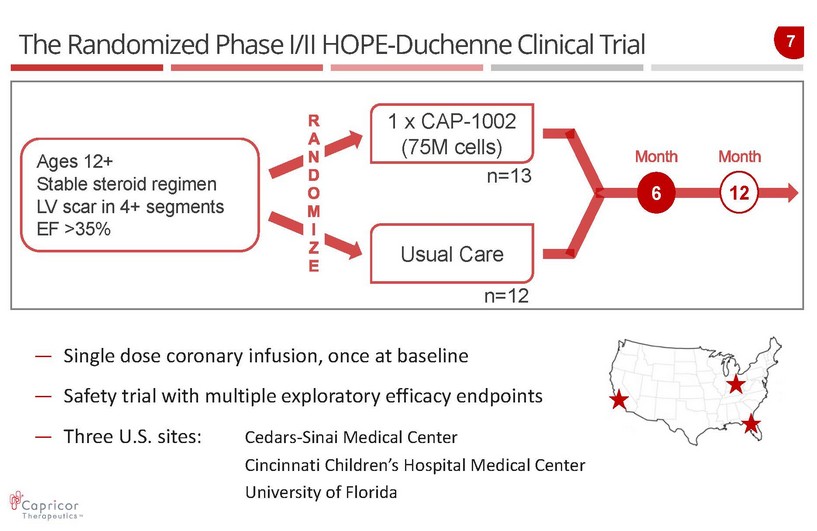
7 The Randomized Phase I/II HOPE - Duchenne Clinical Trial — Single dose coronary infusion, once at baseline — Safety trial with multiple exploratory efficacy endpoints — Three U.S. sites: Cedars - Sinai Medical Center Cincinnati Children’s Hospital Medical Center University of Florida Ages 12+ Stable steroid regimen LV scar in 4+ segments EF >35% 1 x CAP - 1002 (75M cells) Usual Care 6 n=13 n=12 12
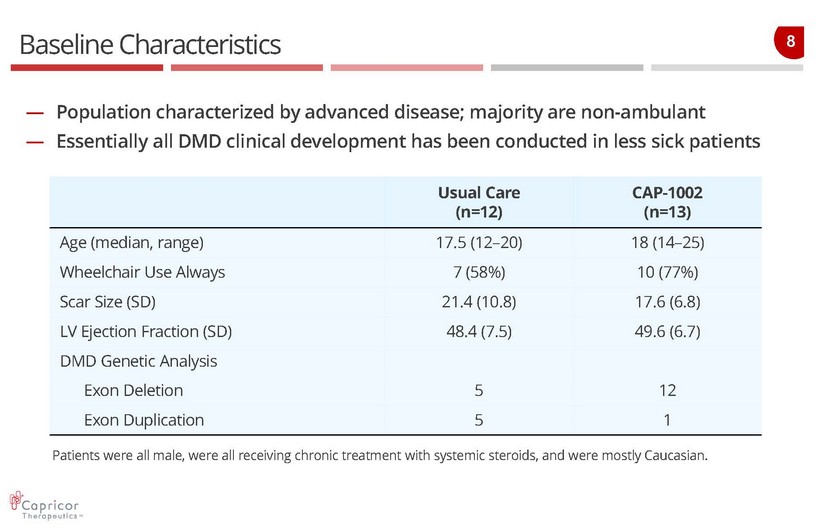
8 Baseline Characteristics — Population characterized by advanced disease; majority are non - ambulant — Essentially all DMD clinical development has been conducted in less sick patients Usual Care (n=12) CAP - 1002 (n=13) Age (median, range) 17.5 (12 – 20) 18 (14 – 25) Wheelchair Use Always 7 (58%) 10 (77%) Scar Size (SD) 21.4 (10.8) 17.6 (6.8) LV Ejection Fraction (SD) 48.4 (7.5) 49.6 (6.7) DMD Genetic Analysis Exon Deletion 5 12 Exon Duplication 5 1 Patients were all male, were all receiving chronic treatment with systemic steroids, and were mostly Caucasian.
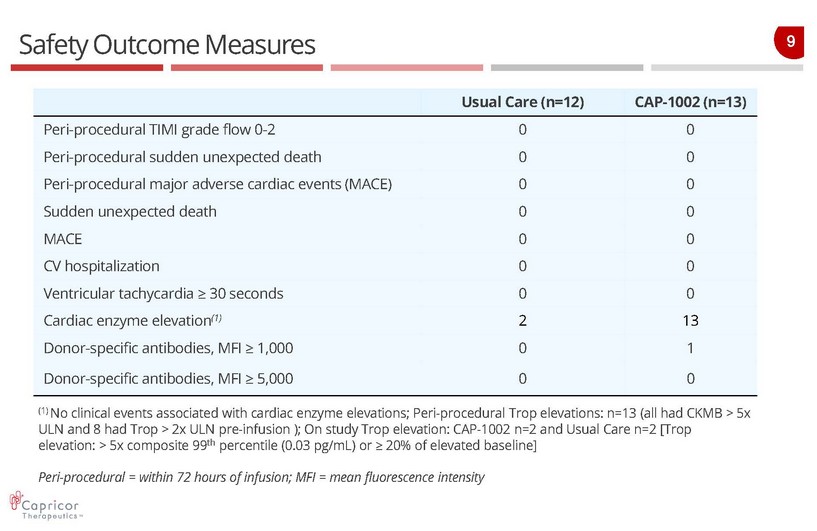
9 Safety Outcome Measures Usual Care (n=12) CAP - 1002 (n=13) Peri - procedural TIMI grade flow 0 - 2 0 0 Peri - procedural sudden unexpected death 0 0 Peri - procedural major adverse cardiac events (MACE) 0 0 Sudden unexpected death 0 0 MACE 0 0 CV hospitalization 0 0 Ventricular tachycardia ≥ 30 seconds 0 0 Cardiac enzyme elevation (1) 2 13 Donor - specific antibodies, MFI ≥ 1,000 0 1 Donor - specific antibodies, MFI ≥ 5,000 0 0 (1) No clinical events associated with cardiac enzyme elevations; Peri - procedural Trop elevations: n=13 (all had CKMB > 5x ULN and 8 had Trop > 2x ULN pre - infusion ); On study Trop elevation: CAP - 1002 n=2 and Usual Care n=2 [ Trop elevation: > 5x composite 99 th percentile (0.03 pg /mL) or ≥ 20% of elevated baseline] Peri - procedural = within 72 hours of infusion; MFI = mean fluorescence intensity
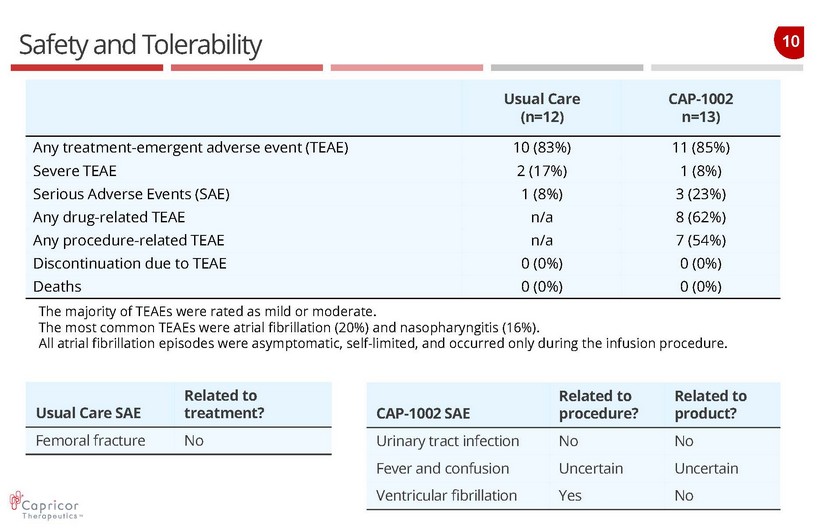
10 Safety and Tolerability Usual Care (n=12) CAP - 1002 n=13) Any treatment - emergent adverse event (TEAE) 10 (83%) 11 (85%) Severe TEAE 2 (17%) 1 (8%) Serious Adverse Events (SAE) 1 (8%) 3 (23%) Any drug - related TEAE n/a 8 (62%) Any procedure - related TEAE n/a 7 (54%) Discontinuation due to TEAE 0 (0%) 0 (0%) Deaths 0 (0%) 0 (0%) The majority of TEAEs were rated as mild or moderate. The most common TEAEs were atrial fibrillation (20%) and nasopharyngitis (16%). All atrial fibrillation episodes were asymptomatic, self - limited, and occurred only during the infusion procedure. Usual Care SAE Related to treatment? Femoral fracture No CAP - 1002 SAE Related to procedure? Related to product? Urinary tract infection No No Fever and confusion Uncertain Uncertain Ventricular fibrillation Yes No
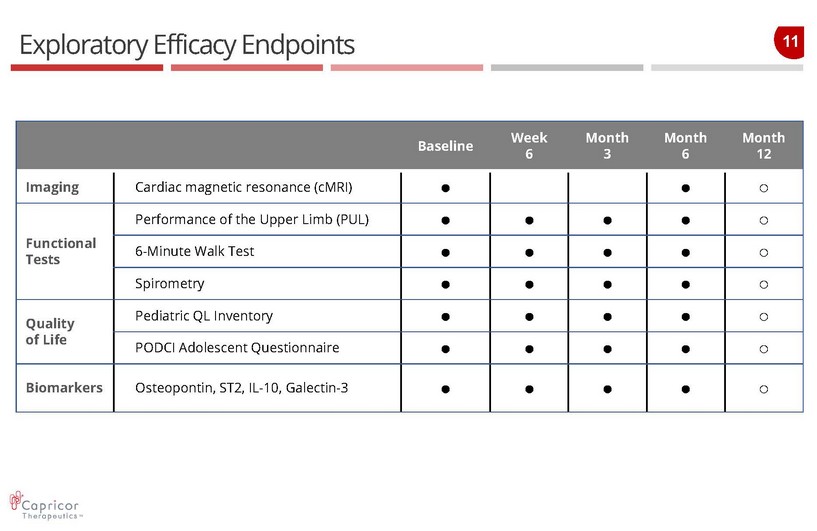
11 Exploratory Efficacy Endpoints Baseline Week 6 Month 3 Month 6 Month 12 Imaging Cardiac magnetic resonance ( cMRI ) ● ● ○ Functional Tests Performance of the Upper Limb (PUL) ● ● ● ● ○ 6 - Minute Walk Test ● ● ● ● ○ Spirometry ● ● ● ● ○ Quality of Life Pediatric QL Inventory ● ● ● ● ○ PODCI Adolescent Questionnaire ● ● ● ● ○ Biomarkers Osteopontin , ST2, IL - 10, Galectin - 3 ● ● ● ● ○
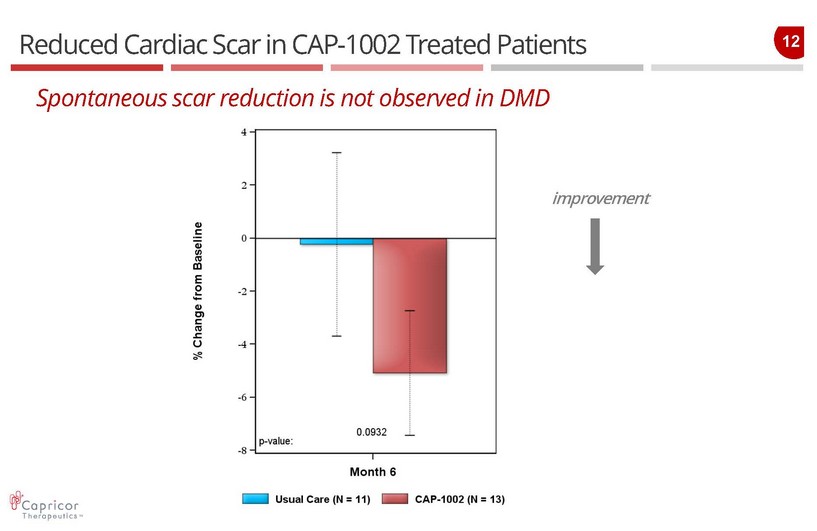
12 Reduced Cardiac Scar in CAP - 1002 Treated Patients Spontaneous scar reduction is not observed in DMD improvement
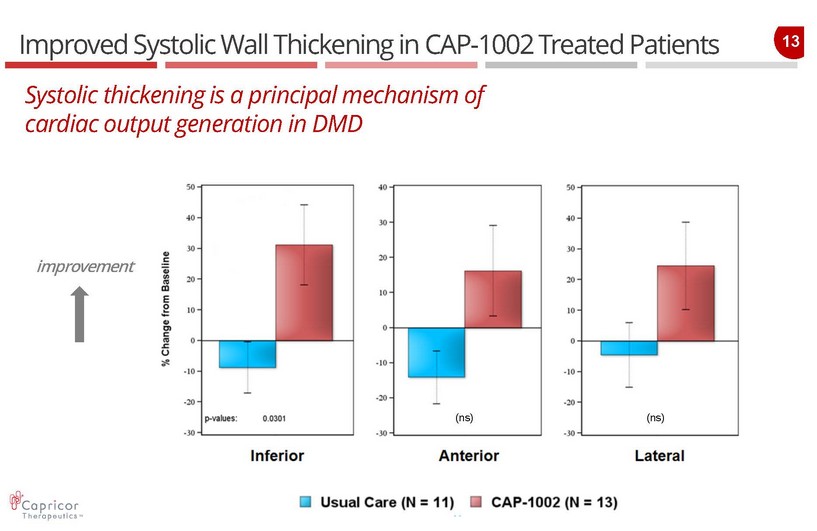
13 Improved Systolic Wall Thickening in CAP - 1002 Treated Patients Systolic thickening is a principal mechanism of cardiac output generation in DMD improvement (ns) (ns)
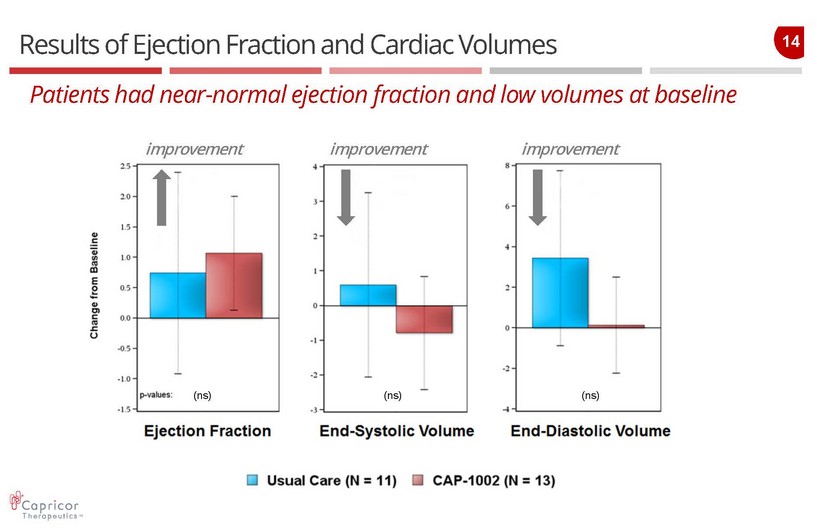
14 Results of Ejection Fraction and Cardiac Volumes Patients had near - normal ejection fraction and low volumes at baseline improvement improvement improvement (ns) (ns) (ns)
15 Performance of the Upper Limb ( PUL) Test — The Performance of the Upper Limb (PUL) test was designed specifically for use in the DMD setting — Evaluates a variety of manual tasks, such as lifting cans, tearing paper, and removing a container lid, which simulate activities of daily living — Boys and young men with advanced DMD have difficulty with such common activities as feeding themselves and brushing their teeth — The PUL was selected for the HOPE trial as a validated test to evaluate skeletal muscle performance in a DMD population with advanced disease (more than two - thirds are non - ambulant)
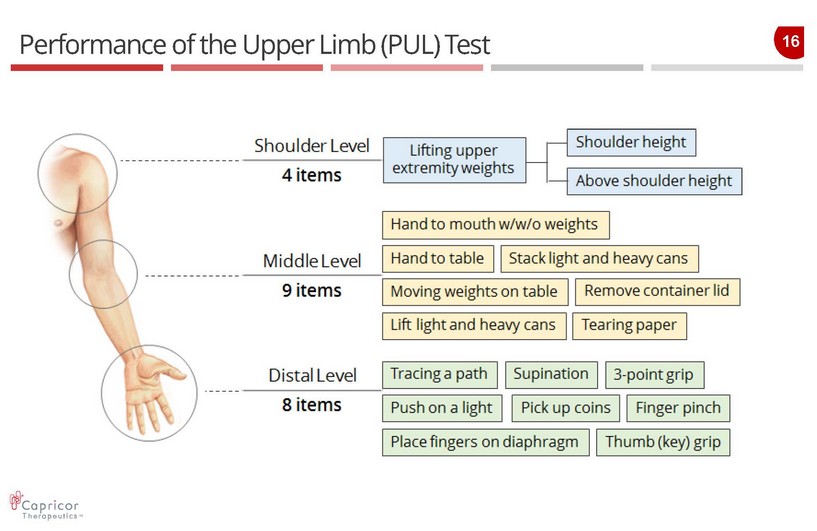
16 Performance of the Upper Limb (PUL ) Test
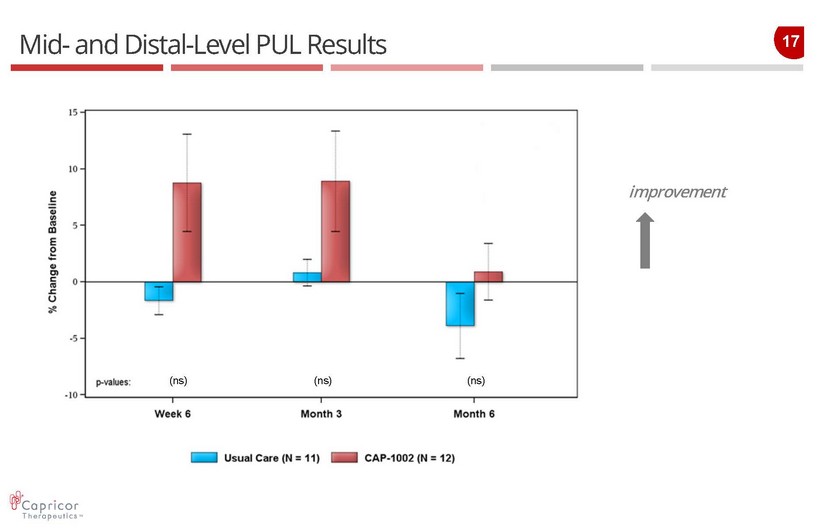
17 Mid - and Distal - Level PUL Results improvement (ns) (ns) (ns)
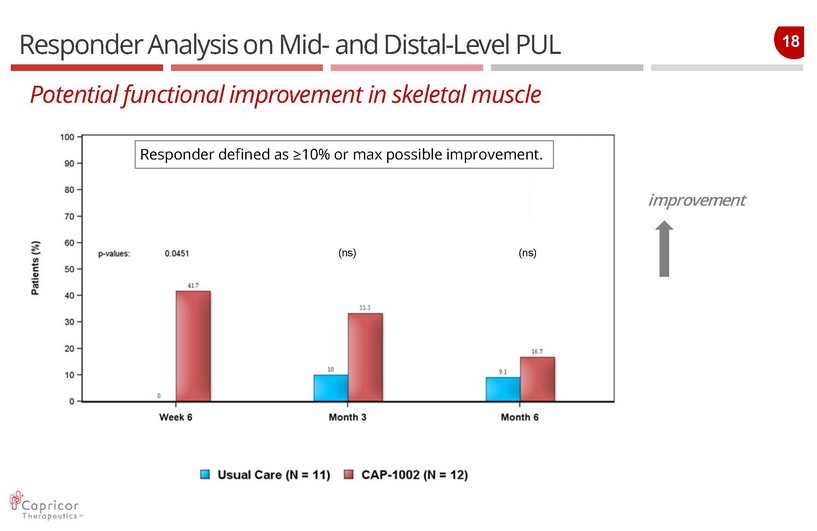
18 Responder Analysis on Mid - and Distal - Level PUL P otential functional improvement in skeletal muscle improvement Responder defined as ≥ 10% or max possible improvement. (ns) (ns)
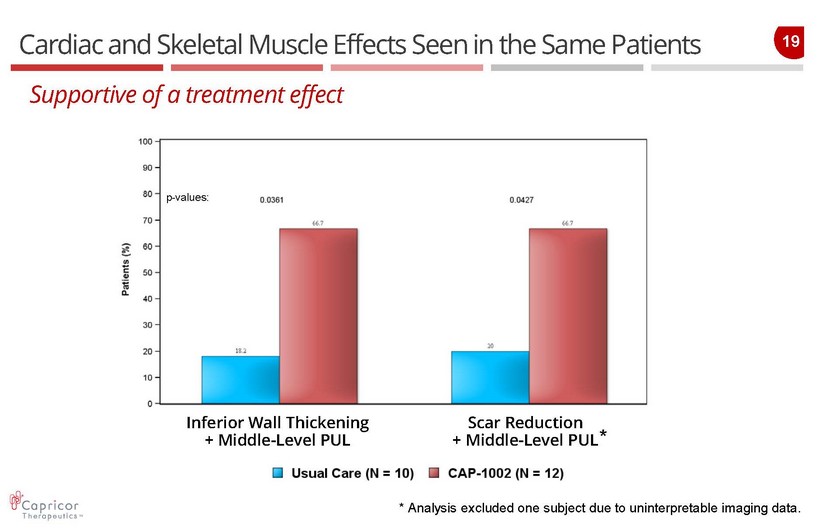
19 Cardiac and Skeletal Muscle Effects Seen in the Same Patients Inferior Wall Thickening + Middle - Level PUL Scar Reduction + Middle - Level PUL Supportive of a treatment effect p - values: * Analysis excluded one subject due to uninterpretable imaging data. *
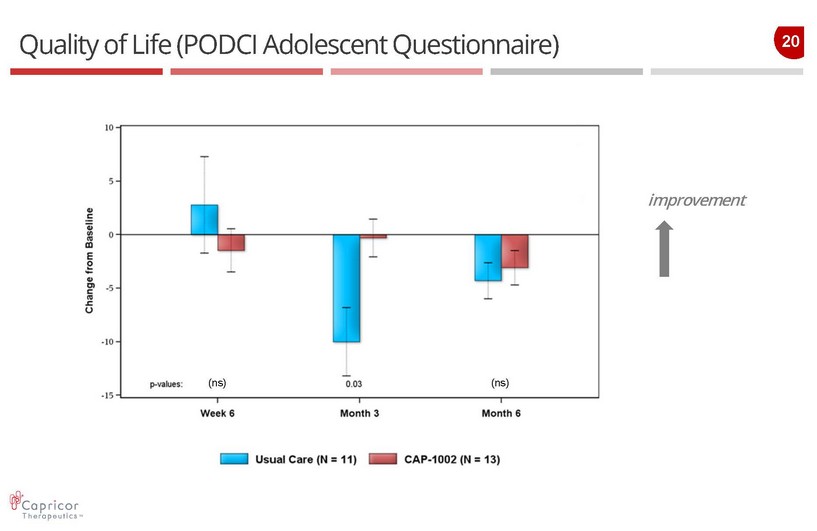
20 Quality of Life (PODCI Adolescent Questionnaire) improvement (ns) (ns)
21 Key Conclusions from Six - Month HOPE Data — Clinical results are in line with preclinical data that illustrate the bioactivity of CAP - 1002 on both cardiac and skeletal muscle in DMD — Exploratory efficacy analyses support the potential benefit of CAP - 1002 to boys and young men with advanced Duchenne muscular dystrophy — CAP - 1002 was generally safe and well - tolerated — Limited duration of effect following a single dose indicates that sustained benefit will require repeat administration

22 Next Steps for Capricor’s Duchenne Muscular Dystrophy Program — Requested FDA meeting to review HOPE data and discuss development toward potential registration — Plan to submit applications for Breakthrough Therapy or Regenerative Medicine Advanced Therapy (RMAT) designations — Repeat dosing via systemic delivery anticipated for next trial ▪ Paradigm for chronic administration to achieve potential long - term benefit ▪ Systemic delivery supported by studies in several models