Exhibit 99.2

Cardiosphere - Derived Cells for the Treatment of Duchenne Cardiomyopathy: Results of the Halt cardiOmyopathy ProgrEssion [HOPE] - Duchenne Trial Ronald Victor, John Jefferies, Michael Taylor, Joao Lima, Rachel Smith, Konstantinos Malliaras, Brian Fedor, Jeff Rudy, Janice Pogoda, Linda Marban, Deborah Ascheim, Eduardo Marban

HOPE - Duchenne Trial sponsored by Capricor, Inc. Ronald Victor, MD • Capricor: Steering Committee, Site PI • Catabasis Pharmaceuticals: Site PI • Coalition Duchenne: Research Grant PI • Eli Lilly: Steering Committee Chair, Global PI, Site PI Disclosures
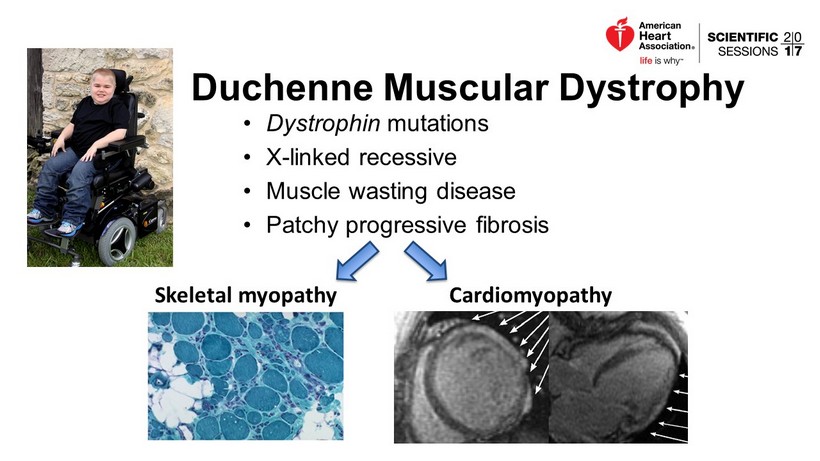
• Dystrophin mutations • X - linked recessive • Muscle wasting disease • Patchy progressive fibrosis Duchenne Muscular Dystrophy Skeletal myopathy Cardiomyopathy
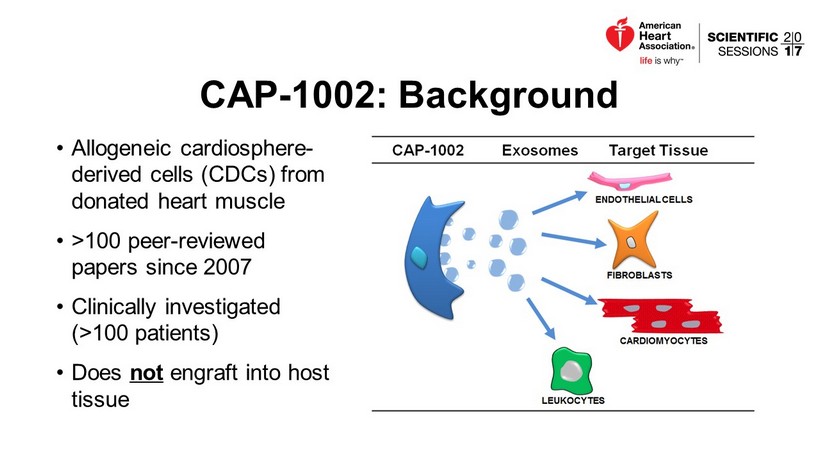
CAP - 1002: Background • Allogeneic cardiosphere - derived cells (CDCs) from donated heart muscle • >100 peer - reviewed papers since 2007 • Clinically investigated ( >100 patients) • Does not engraft into host tissue
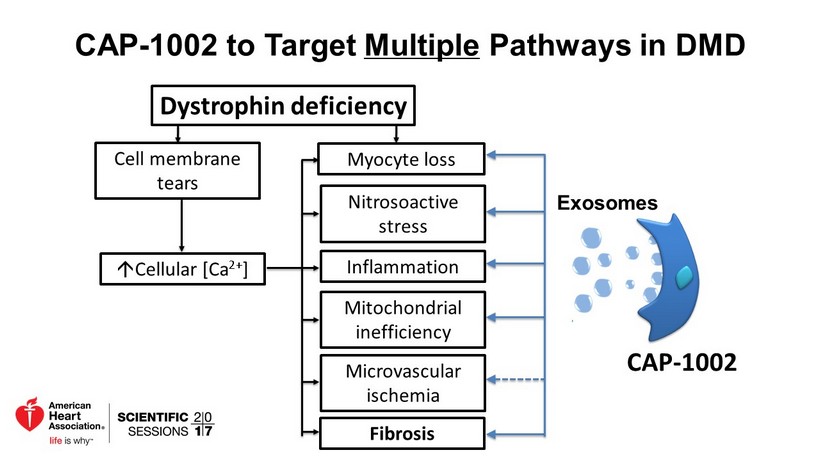
CAP - 1002 to Target Multiple Pathways in DMD Dystrophin deficiency Cell membrane tears; Cellular [Ca 2+ ] Myocyte loss Nitrosoactive stress Inflammation Mitochondrial inefficiency Fibrosis Microvascular ischemia CAP - 1002 Exosomes

Improved exercise capacity Intracardiac CDCs in mdx Mouse Model of DMD Improved cardiac function Aminzadeh et al., 2017. Preprint: a http ://biorxiv.org/content/early/2017/04/20/128900 The challenge: clinical translation…
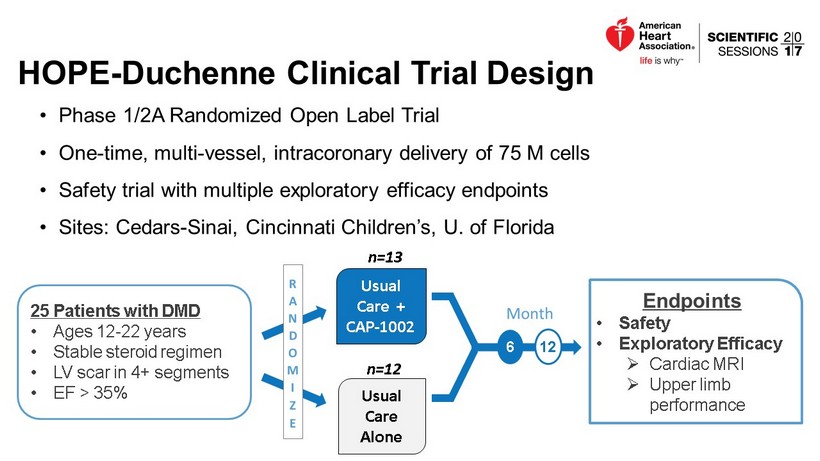
HOPE - Duchenne Clinical Trial Design • Phase 1/2A Randomized Open Label Trial • One - time, multi - vessel, intracoronary delivery of 75 M cells • Safety trial with multiple exploratory efficacy endpoints • Sites: Cedars - Sinai, Cincinnati Children’s, U. of Florida Endpoints • Safety • Exploratory Efficacy » Cardiac MRI » Upper limb performance 6 12 25 Patients with DMD • Ages 12 - 22 years • Stable steroid regimen • LV scar in 4+ segments • EF > 35% Usual Care Alone Usual Care + CAP - 1002 n=13 n=12
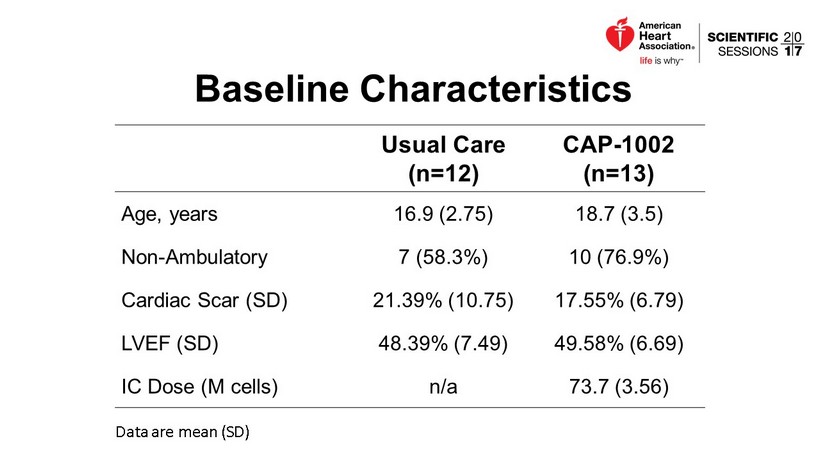
Baseline Characteristics Usual Care (n=12) CAP - 1002 (n=13) Age , years 16.9 (2.75) 18.7 (3.5) Non - Ambulatory 7 (58.3%) 10 (76.9%) Cardiac Scar (SD) 21.39% (10.75) 17.55% (6.79) LVEF (SD) 48.39% (7.49) 49.58% (6.69) IC Dose (M cells) n/a 73.7 (3.56) Data are mean (SD)
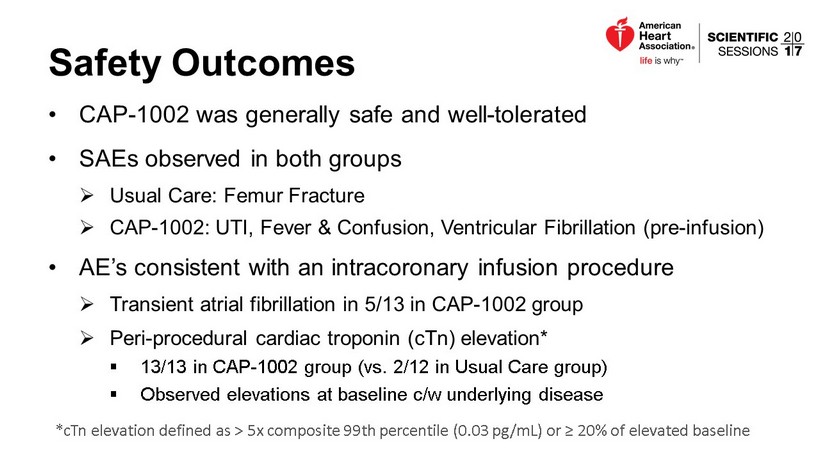
Safety Outcomes • CAP - 1002 was generally safe and well - tolerated • SAEs observed in both groups » Usual Care: Femur Fracture » CAP - 1002: UTI, Fever & Confusion, Ventricular Fibrillation (pre - infusion) • AE’s consistent with an intracoronary infusion procedure » Transient atrial fibrillation in 5/13 in CAP - 1002 group » Peri - procedural cardiac troponin ( cTn ) elevation* ▪ 13/13 in CAP - 1002 group (vs. 2/12 in Usual Care group) ▪ Observed elevations at baseline c/w underlying disease * cTn elevation defined as > 5x composite 99th percentile (0.03 pg /mL) or ≥ 20% of elevated baseline
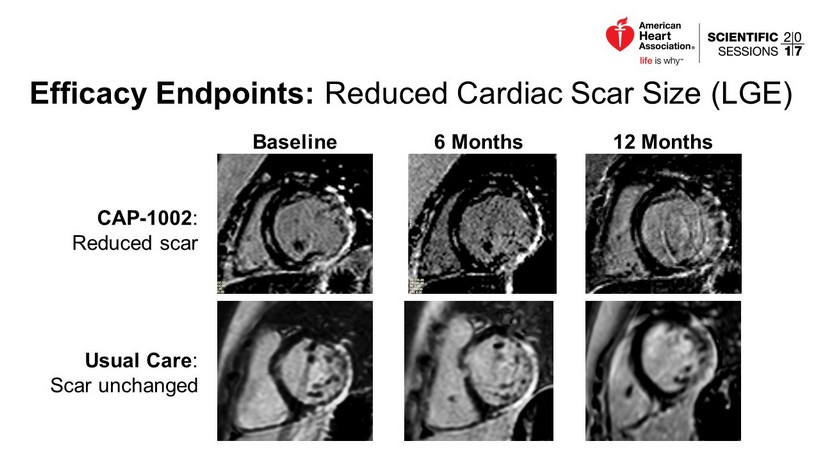
Efficacy Endpoints: Reduced Cardiac Scar Size (LGE) CAP - 1002 : Reduced scar Usual Care : Scar unchanged Baseline 6 Months 12 Months

Reduced Myocardial Scar by Cardiac MRI • Blinded analysis by core lab • By Month 12, scar increased in the Usual Care group but decreased in the CAP - 1002 group » 11.9% group difference in change score (p=0.03) • Decreased scar is counter to the natural history of DMD. % Change from Baseline p=0.09 p=0.03 p=0.09

Improved Regional Systolic Wall Thickening • No effect detected in overall EF • Most evidence of improvement in inferior wall • Similar trend in anterior wall • Lesser trends in lateral and septal walls » Consistent with natural history of scar progression in DMD Inferior → Anterior → Lateral → Septal INFERIOR WALL ANTERIOR WALL % change from baseline p=0.04 p=0.09 p=0.09 p=0.10 p=0.54 p=0.09
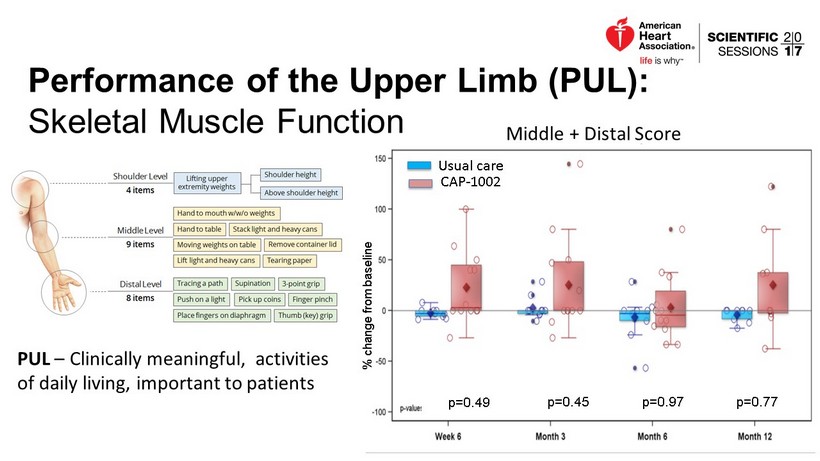
Performance of the Upper Limb (PUL): Skeletal Muscle Function PUL – Clinically meaningful, activities of daily living, important to patients % change from baseline Middle + Distal Score p=0.49 p=0.77 p=0.97 p=0.45 Usual care CAP - 1002

p=0.10 p=0.22 p=0.49 p=0.22 Patients (%) PUL: Post Hoc Responder Analysis • Middle + Distal PUL • Subgroup of patients with baseline middle + distal PUL score < 55 (maximum = 58) • Responder defined as ≥ 10% from baseline or max possible improvement (n=9) (n=4) (n=9) (n=9) (n=9) (n=5) (n=6) (n=4)
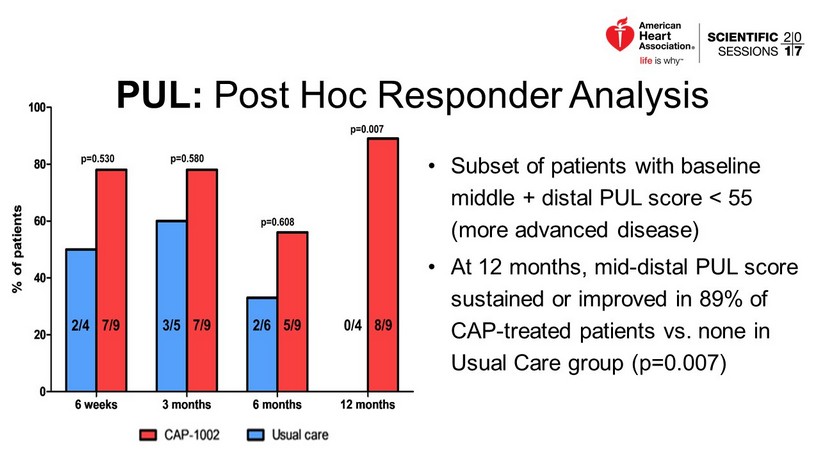
• Subset of patients with baseline middle + distal PUL score < 55 (more advanced disease) • At 12 months, mid - distal PUL score sustained or improved in 89% of CAP - treated patients vs. none in Usual Care group (p=0.007) PUL: Post Hoc Responder Analysis
Conclusions • CAP - 1002 delivered via IC infusion was generally safe and well - tolerated • These early clinical data are consistent with preclinical studies showing CAP - 1002 benefits both cardiac and skeletal muscle in DMD. • Exploratory efficacy analyses signal a potential benefit of CAP - 1002 for patients with advanced DMD. Planned “ HOPE - 2 ” trial: » Similar patient population » Intravenous delivery Q3 months » Evaluate skeletal and cardiac muscle function » Enrollment to begin in 1Q 2018
Acknowledgements • Funded in part by the California Institute for Regenerative Medicine (CIRM) • Coalition Duchenne • CureDuchenne • Parent Project Muscular Dystrophy • Site Principal Investigators » John Jefferies » Barry Byrne • Site Interventional Cardiologists » Raj Makkar » Bryan Goldstein » James Fudge