
Exhibit 99.3

Intracoronary ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR): A Randomized, placebo - controlled, double - blind trial Timothy D. Henry, Dean J. Kereiakes, Glenn Kowalchuk, Frank Aguirre, Konstantinos Malliaris, Anthony DeMaria, Gary Francis, Thomas J. Povsic, Richard Schatz, Jay H. Traverse, Tarun Chakravarty, Janice Pogoda, Paula Williams, Jeff Rudy, Rachel D. Smith, Linda Marbán , Deborah D. Ascheim, Eduardo Marbán , Raj R. Makkar

Disclosures Trial Sponsors : Capricor, Inc.; NIH/NHLBI (Phase I - 1RC3HL103356 ARRA grant ) Funded in part by California Institute for Regenerative Medicine (CIRM; Phase 2) Co - PIs Timothy D. Henry & Raj Makkar Steering Committee Anthony DeMaria, MD – Chair Gary S. Francis, MD Frank Aguirre, MD Thomas Povsic, MD, PhD Richard Schatz, MD Eduardo Marbán , MD, PhD – Advisor
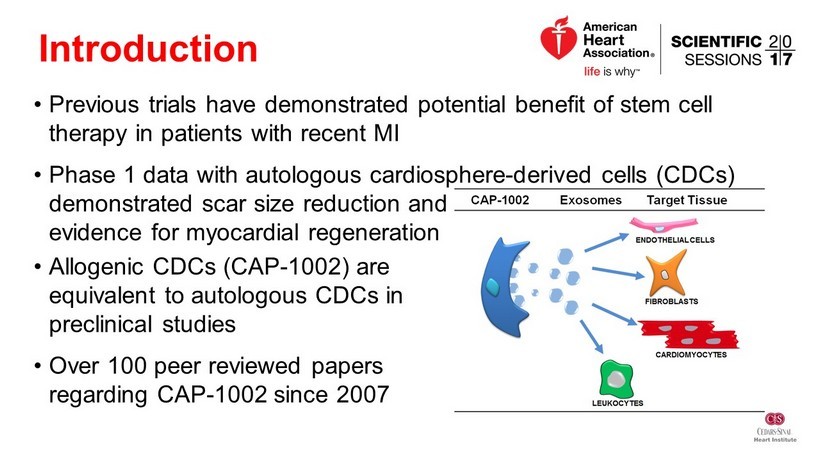
Introduction • Previous trials have demonstrated potential benefit of stem cell therapy in patients with recent MI • Phase 1 data with autologous cardiosphere - derived cells (CDCs) demonstrated scar size reduction and evidence for myocardial regeneration • Allogenic CDCs (CAP - 1002) are equivalent to autologous CDCs in preclinical studies • Over 100 peer reviewed papers regarding CAP - 1002 since 2007
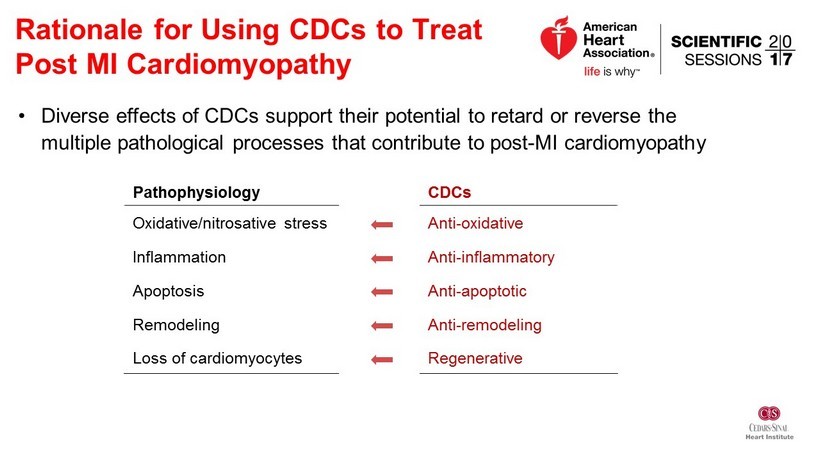
Rationale for Using CDCs to Treat Post MI Cardiomyopathy Pathophysiology CDCs Oxidative/ nitrosative stress Anti - oxidative Inflammation Anti - inflammatory Apoptosis Anti - apoptotic Remodeling Anti - remodeling Loss of cardiomyocytes Regenerative • Diverse effects of CDCs support their potential to retard or reverse the multiple pathological processes that contribute to post - MI cardiomyopathy
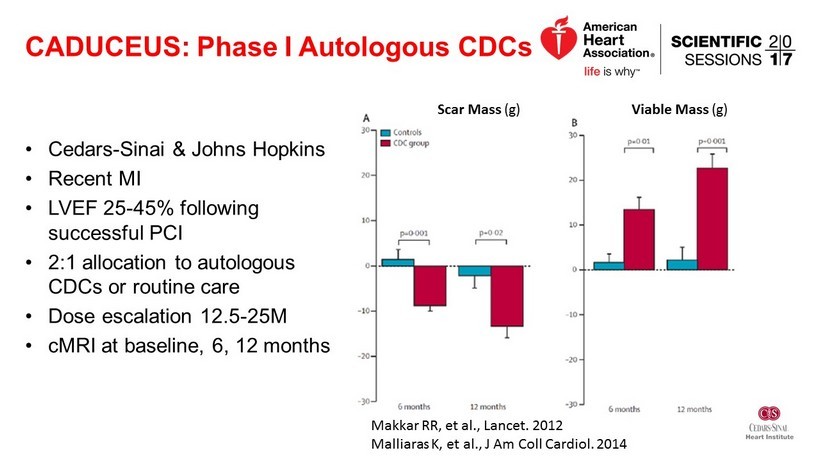
CADUCEUS: Phase I Autologous CDCs • Cedars - Sinai & Johns Hopkins • Recent MI • LVEF 25 - 45% following successful PCI • 2:1 allocation to autologous CDCs or routine care • Dose escalation 12.5 - 25M • cMRI at baseline, 6, 12 months Scar Mass (g) Viable Mass (g) Makkar RR, et al., Lancet. 2012 Malliaras K, et al., J Am Coll Cardiol . 2014
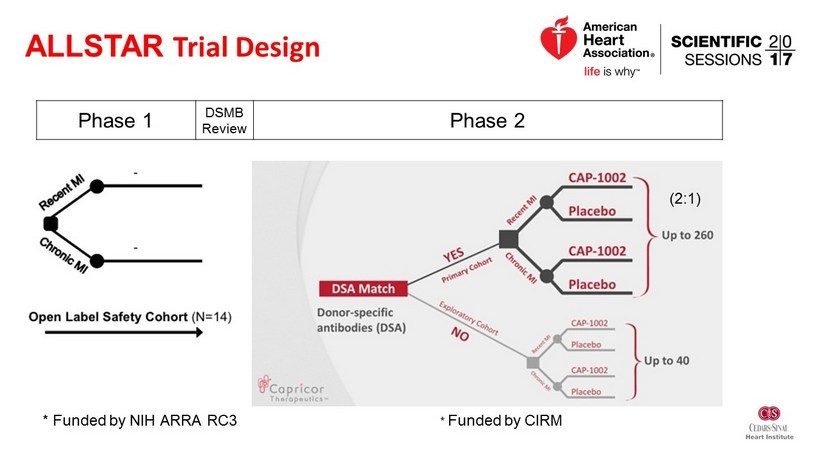
* Funded by CIRM ALLSTAR Trial Design * Funded by NIH ARRA RC3 Phase 1 DSMB Review Phase 2 (2:1)
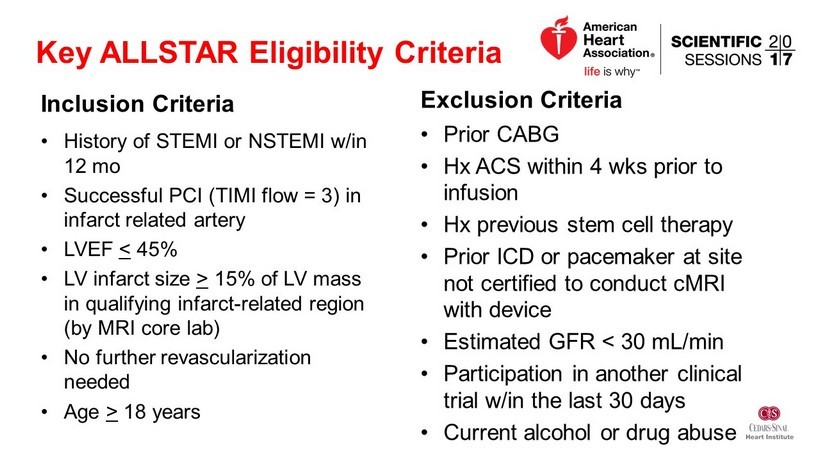
Key ALLSTAR Eligibility Criteria Inclusion Criteria • History of STEMI or NSTEMI w/in 12 mo • Successful PCI (TIMI flow = 3) in infarct related artery • LVEF < 45% • LV infarct size > 15% of LV mass in qualifying infarct - related region (by MRI core lab) • No further revascularization needed • Age > 18 years Exclusion Criteria • Prior CABG • Hx ACS within 4 wks prior to infusion • Hx previous stem cell therapy • Prior ICD or pacemaker at site not certified to conduct cMRI with device • Estimated GFR < 30 mL/min • Participation in another clinical trial w/in the last 30 days • Current alcohol or drug abuse
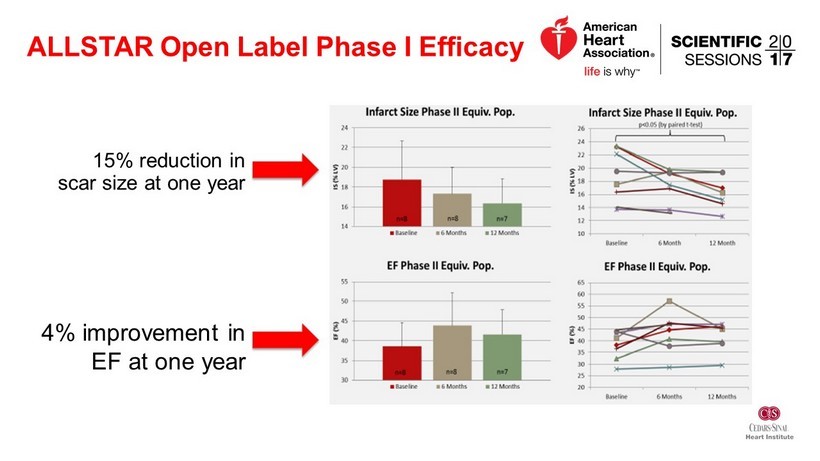
ALLSTAR Open Label Phase I Efficacy 15% reduction in scar size at one year 4% improvement in EF at one year
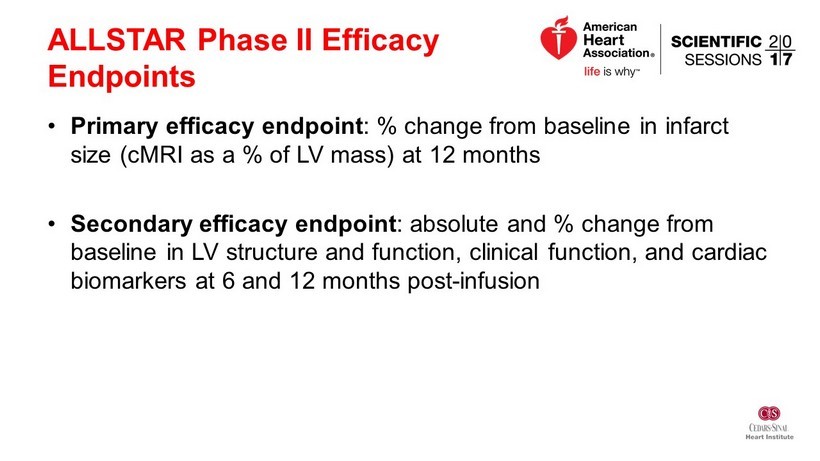
ALLSTAR Phase II Efficacy Endpoints • Primary efficacy endpoint : % change from baseline in infarct size ( cMRI as a % of LV mass) at 12 months • Secondary efficacy endpoint : absolute and % change from baseline in LV structure and function, clinical function, and cardiac biomarkers at 6 and 12 months post - infusion
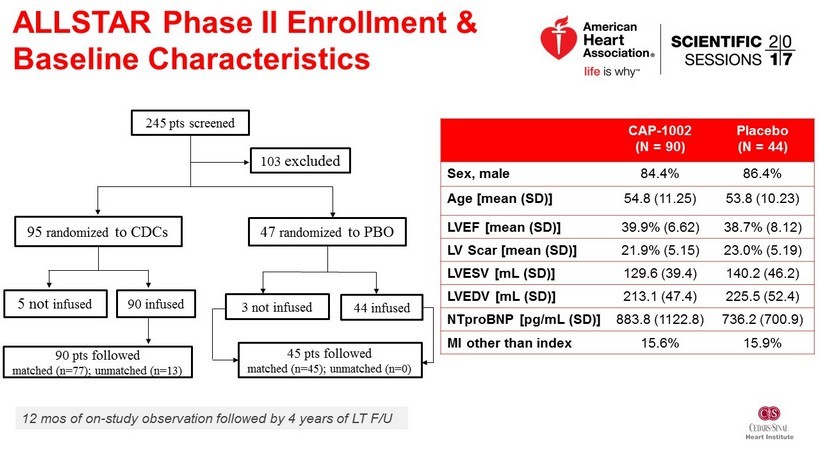
245 pts screened 95 randomized to CDCs 47 randomized to PBO 90 pts followed matched (n=77); unmatched (n=13) 45 pts followed matched (n=45); unmatched (n=0) 103 excluded 5 not infused 90 infused 44 infused CAP - 1002 (N = 90) Placebo (N = 44) Sex, male 84.4% 86.4% Age [mean (SD)] 54.8 (11.25) 53.8 (10.23) LVEF [mean (SD)] 39.9% (6.62) 38.7% (8.12) LV Scar [mean (SD)] 21.9% (5.15) 23.0% (5.19) LVESV [mL (SD)] 129.6 (39.4) 140.2 (46.2) LVEDV [mL (SD)] 213.1 (47.4) 225.5 (52.4) NTproBNP [ pg /mL (SD)] 883.8 (1122.8) 736.2 (700.9) MI other than index 15.6% 15.9% ALLSTAR Phase II Enrollment & Baseline Characteristics 12 mos of on - study observation followed by 4 years of LT F/U 3 not infused
Interim Analysis • Pre - specified interim analysis – after all subjects observed ≥ 6 months – All data for 134 treated subjects (Primary Cohort, n=121; Exploratory Cohort, n=13) • Given low probability that treatment effect would be observed in primary 12 month efficacy analysis, all subjects were transitioned to annual follow - up
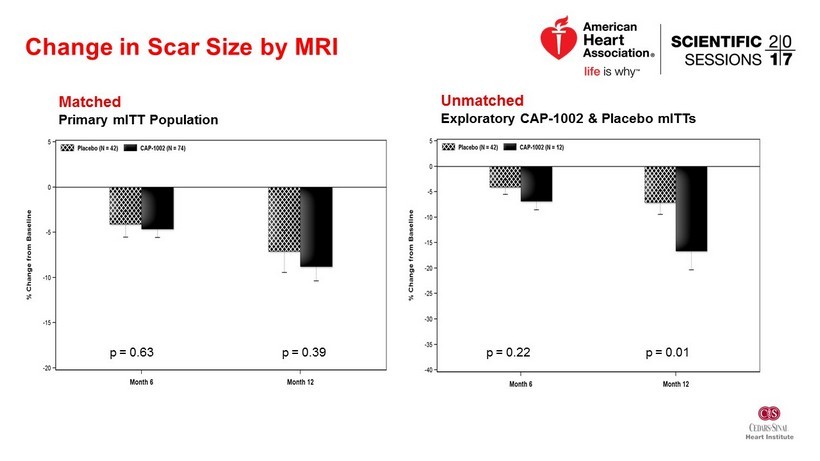
Change in Scar Size by MRI Matched Primary mITT Population p = 0.63 p = 0.39 p = 0.39 p = 0.63 Unmatched Exploratory CAP - 1002 & Placebo mITTs p = 0.22 p = 0.01
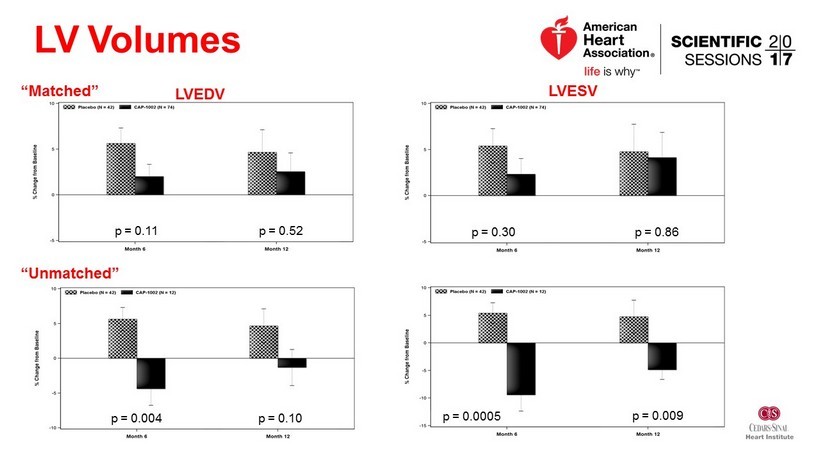
LV Volumes “Matched” LVESV LVEDV p = 0.11 p = 0.52 p = 0.30 p = 0.86 “Unmatched” p = 0.004 p = 0.10 p = 0.0005 p = 0.009
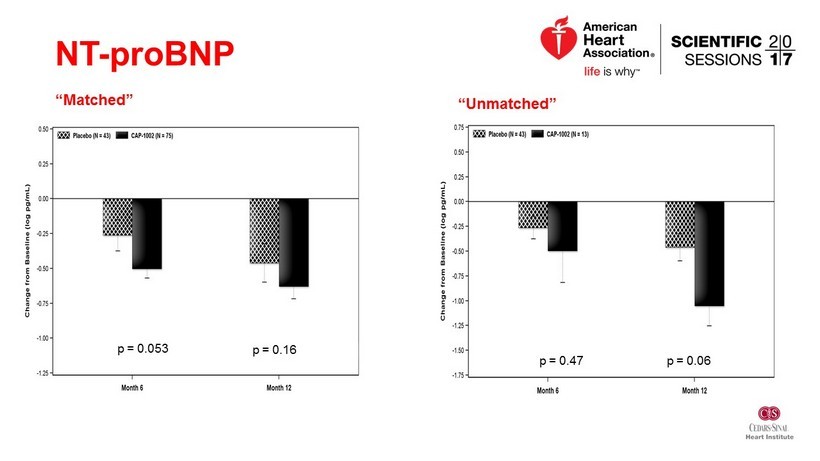
NT - proBNP “Matched” p = 0.053 p = 0.16 “Unmatched” p = 0.47 p = 0.06
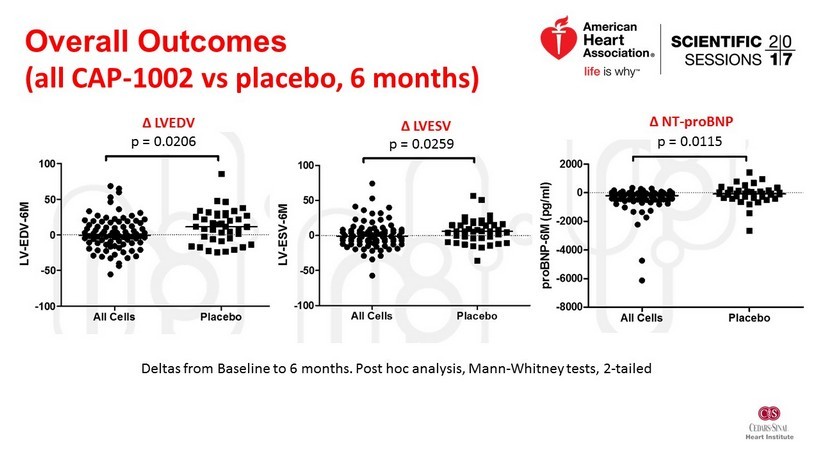
Overall Outcomes (all CAP - 1002 vs placebo, 6 months) Deltas from Baseline to 6 months. Post hoc analysis, Mann - Whitney tests, 2 - tailed Δ LVEDV Δ LVESV Δ NT - proBNP p = 0.0206 p = 0.0259 p = 0.0115
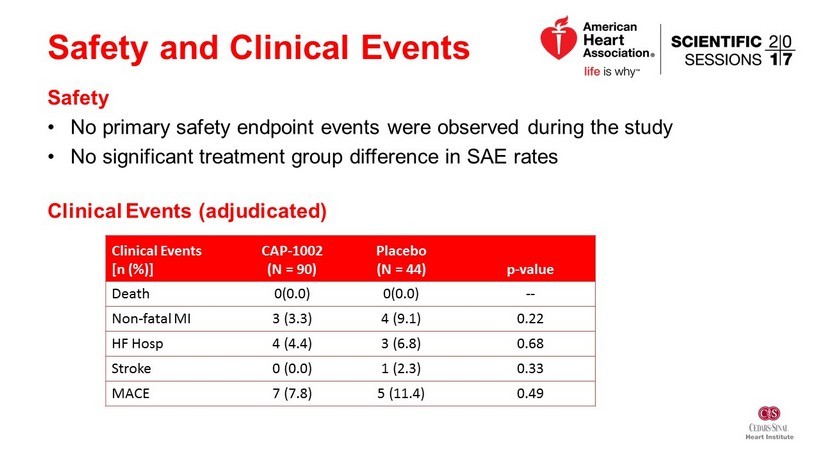
Safety and Clinical Events Safety • No primary safety endpoint events were observed during the study • No significant treatment group difference in SAE rates Clinical Events (adjudicated) Clinical Events [n (%)] CAP - 1002 (N = 90) Placebo (N = 44) p - value Death 0(0.0) 0(0.0) -- Non - fatal MI 3 (3.3) 4 (9.1) 0.22 HF Hosp 4 (4.4) 3 (6.8) 0.68 Stroke 0 (0.0) 1 (2.3) 0.33 MACE 7 (7.8) 5 (11.4) 0.49
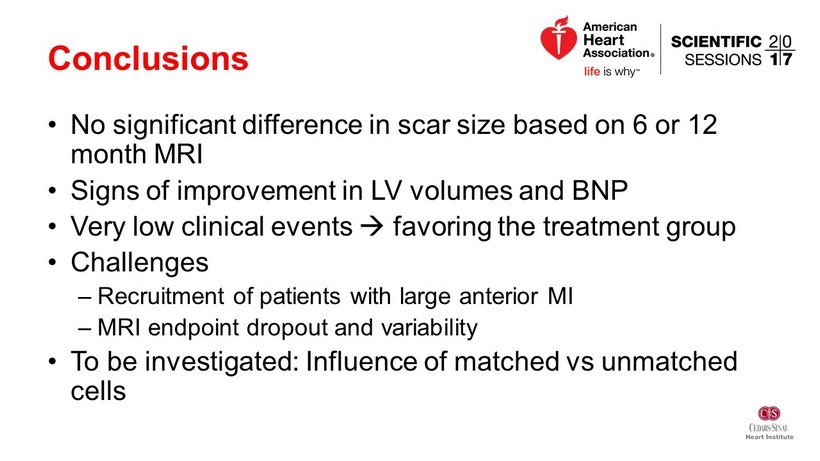
Conclusions • No significant difference in scar size based on 6 or 12 month MRI • Signs of improvement in LV volumes and BNP • Very low clinical events - favoring the treatment group • Challenges – Recruitment of patients with large anterior MI – MRI endpoint dropout and variability • To be investigated: Influence of matched vs unmatched cells
Acknowledgments • Cedars - Sinai Medical Center – R. Makkar & T. Henry (17)* • Linder Center for Research & Education – D. Kereiakes (11) • Prairie Education and Research – F. Aguirre (11) • Sanger Heart & Vascular Institute – G. Kowalchuk (10) • Scripps Green Hospital – R. Schatz (9)* • Minneapolis Heart Institute – J. Traverse (6)* • Unv. Massachusetts Memorial – J. Rade (6) • Unv. Pittsburgh Medical Center – C. Toma (6) • Unv. Utah – C. Selzman (6) • Unv. Kentucky – A. Abdel - Latif (5) • Austin Heart – R. Gammon (4) • Ohio Health Research Institute – C. Sanchez (4) • SUNY Buffalo – V. Iyer (4) • Rush University Medical Center – G. Schaer (3) • Duke University Hospital – T. Povsic (3) • MHVI - J. Chambers (3) • Swedish Medical Center/ H & V Research – P. Huang (3) • The Ohio State University – K. Boudoulas (3) • UVMC – M. Watkins (3) • Aurora Healthcare – T. Bajwa(2) • Cardiology PC – F. Mendelsohn (2) • Heart Center Research/Huntsville – A. Vasquez (2) • Michigan CV Institute – S. Kassas (2) • Ottawa Heart Institute – M. Le May (2) • Unv. Florida Health Shands Hospital – C. Pepine (2) • Lenox Hill Hospital – V. Singh (1) • NC Heart – J. Zidar (1) • Unv. Pennsylvania – S. Khandhar (1) • Unv. Texas/Mem Hermann – H.V. Anderson (1) • Unv. Washington – C. Don (1) *Participated in Phase I