
Exhibit 99.1

A Study of CAP - 1002 in Ambulatory and Non - Ambulatory Patients with Duchenne Muscular Dystrophy [HOPE - 2] Updated Results from the Interim Analysis Presented at the 24 th International Annual Congress of the World Muscle Society 1 October 7, 2019 Conference Call NASDAQ: CAPR

Forward - Looking Statements 2 Statements in this press release regarding the efficacy, safety, and intended utilization of Capricor's product candidates ; the initiation, conduct, size, timing and results of discovery efforts and clinical trials ; the pace of enrollment of clinical trials ; plans regarding regulatory filings, future research and clinical trials ; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market ; plans regarding current and future collaborative activities and the ownership of commercial rights ; scope, duration, validity and enforceability of intellectual property rights ; future royalty streams, revenue projections ; expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings, and any other statements about Capricor's management team's future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2018 as filed with the Securities and Exchange Commission on March 29 , 2019 , and as amended by its Amendment No . 1 to Annual Report on Form 10 - K/A filed with the Securities and Exchange Commission on April 1 , 2019 , in its Quarterly Report on Form 10 - Q for the quarterly period ended June 30 , 2019 , as filed with the Securities and Exchange Commission on August 8 , 2019 , and in its Registration Statement on Form S - 3 as filed with the Securities and Exchange Commission on October 24 , 2018 , and as amended by its Amendment No . 1 to Form S - 3 filed with the Securities and Exchange Commission on July 17 , 2019 , together with prospectus supplements thereto . All forward - looking statements in this press release are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward - looking statements . CAP - 1002 is an Investigational New Drug and is not approved for any indications . CAP - 2003 has not yet been approved for clinical investigation .
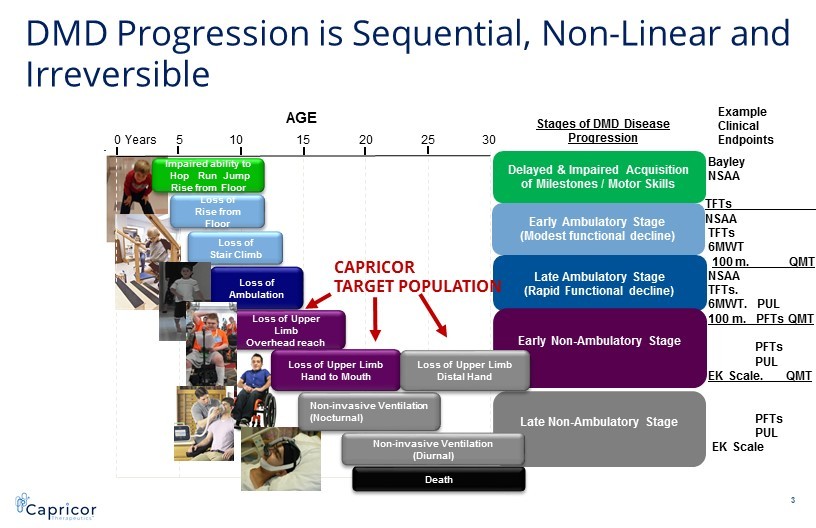
0 5 10 15 20 25 30 Years Loss of Ambulation Death Impaired ability to Hop Run Jump Rise from Floor Loss of Rise from Floor Loss of Stair Climb Stages of DMD Disease Progression Loss of Upper Limb Overhead reach 3 Late Ambulatory Stage (Rapid Functional decline) Delayed & Impaired Acquisition of Milestones / Motor Skills Early Ambulatory Stage (Modest functional decline) Late Non - Ambulatory Stage Early Non - Ambulatory Stage Loss of Upper Limb Hand to Mouth Non - invasive Ventilation (Nocturnal) Loss of Upper Limb Distal Hand Non - invasive Ventilation (Diurnal) AGE Bayley NSAA TFTs____________ NSAA TFTs 6MWT 100 m. QMT NSAA TFTs. 6MWT. PUL 100 m. PFTs QMT PFTs PUL EK Scale. QMT PFTs PUL EK Scale Example Clinical Endpoints DMD Progression is Sequential, Non - Linear and Irreversible CAPRICOR TARGET POPULATION
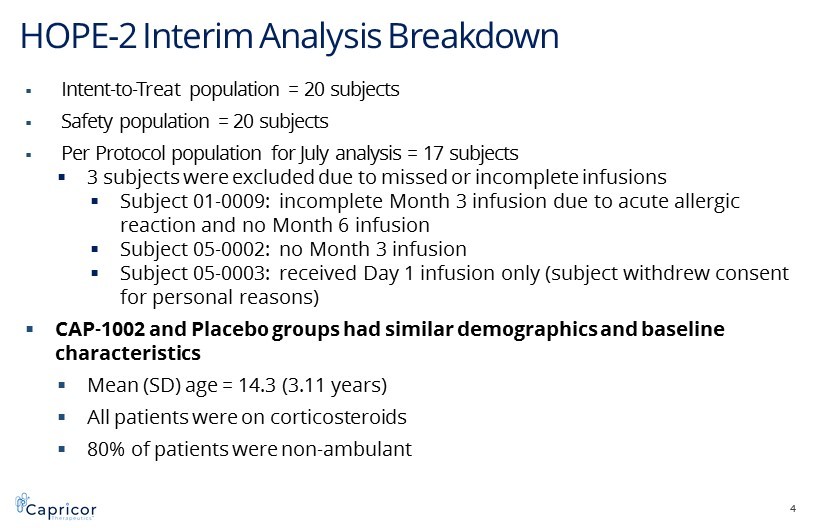
HOPE - 2 Interim Analysis Breakdown 4 ▪ Intent - to - Treat population = 20 subjects ▪ Safety population = 20 subjects ▪ Per Protocol population for July analysis = 17 subjects ▪ 3 subjects were excluded due to missed or incomplete infusions ▪ Subject 01 - 0009: incomplete Month 3 infusion due to acute allergic reaction and no Month 6 infusion ▪ Subject 05 - 0002: no Month 3 infusion ▪ Subject 05 - 0003: received Day 1 infusion only (subject withdrew consent for personal reasons) ▪ CAP - 1002 and Placebo groups had similar demographics and baseline characteristics ▪ Mean (SD) age = 14.3 (3.11 years) ▪ All patients were on corticosteroids ▪ 80% of patients were non - ambulant
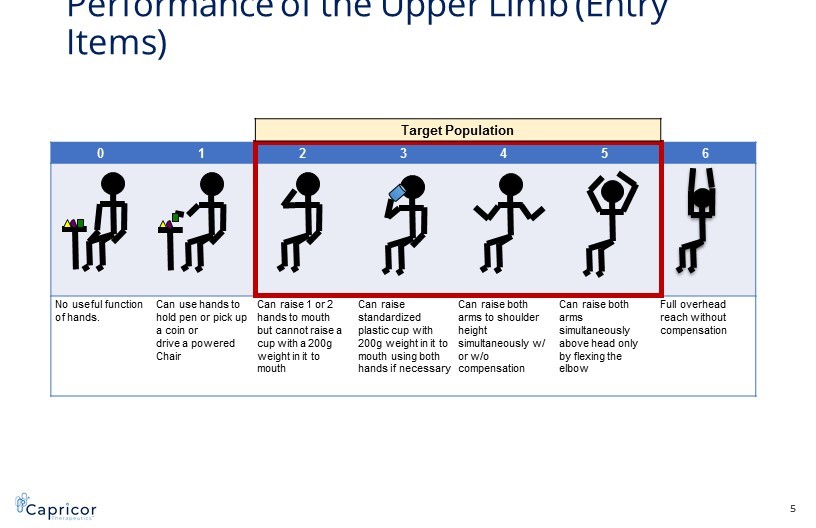
0 1 2 3 4 5 6 No useful function of hands. Can use hands to hold pen or pick up a coin or drive a powered Chair Can raise 1 or 2 hands to mouth but cannot raise a cup with a 200g weight in it to mouth Can raise standardized plastic cup with 200g weight in it to mouth using both hands if necessary Can raise both arms to shoulder height simultaneously w/ or w/o compensation Can raise both arms simultaneously above head only by flexing the elbow Full overhead reach without compensation Performance of the Upper Limb (Entry Items) Target Population 5
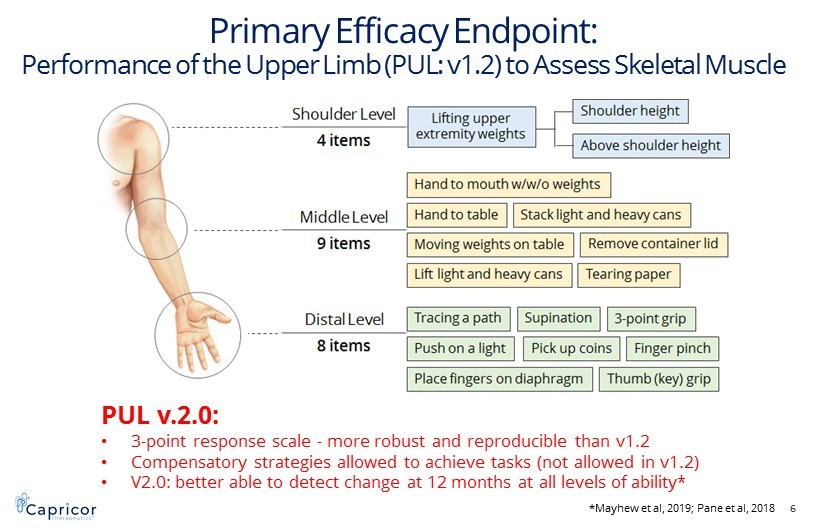
Primary Efficacy Endpoint: PUL Assessment to Assess Skeletal Muscle Primary Efficacy Endpoint: Performance of the Upper Limb (PUL: v1.2) to Assess Skeletal Muscle 6 PUL v.2.0: • 3 - point response scale - more robust and reproducible than v1.2 • Compensatory strategies allowed to achieve tasks (not allowed in v1.2) • V2.0: better able to detect change at 12 months at all levels of ability* *Mayhew et al, 2019; Pane et al, 2018
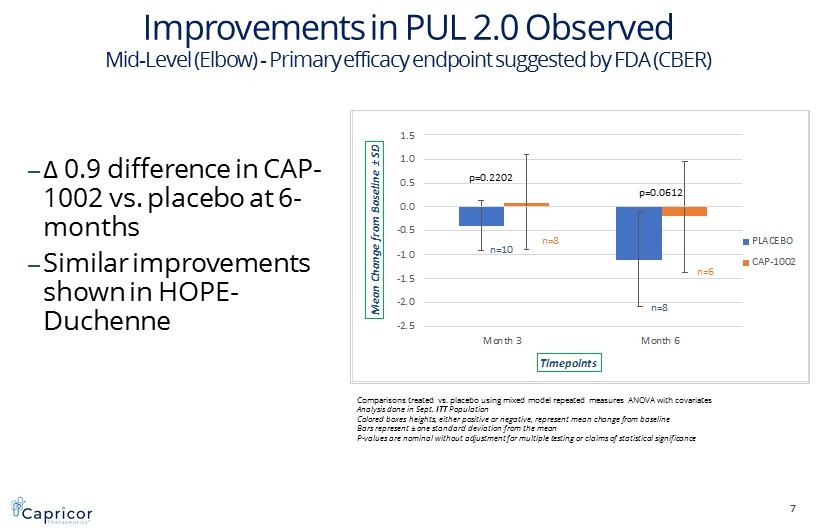
7 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints Mid-Level Performance of the Upper Limb 2.0 PLACEBO CAP-1002 p=0.2202 p=0.0612 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in Sept. ITT Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance Improvements in PUL 2.0 Observed Mid - Level (Elbow) - P rimary efficacy endpoint suggested by FDA (CBER) n=10 n=8 n=8 n=6 ‒ Δ 0.9 difference in CAP - 1002 vs. placebo at 6 - months ‒ Similar improvements shown in HOPE - Duchenne
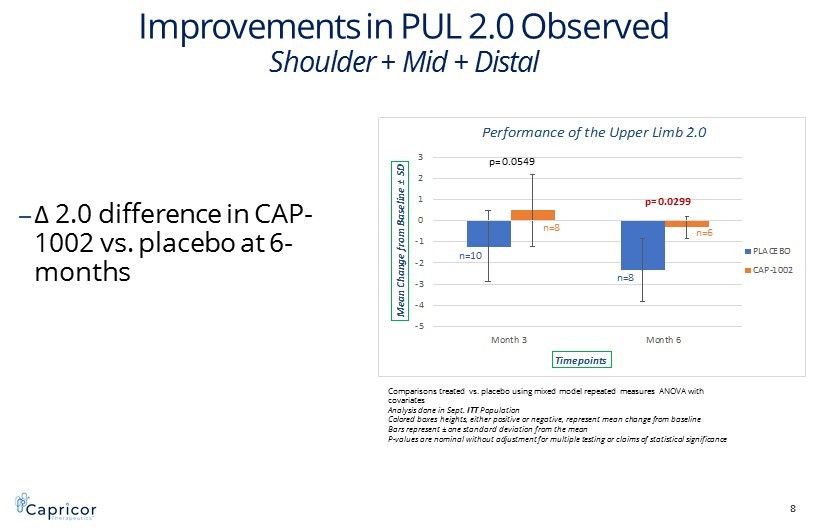
-5 -4 -3 -2 -1 0 1 2 3 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints Performance of the Upper Limb 2.0 PLACEBO CAP-1002 Improvements in PUL 2.0 Observed Shoulder + Mid + Distal ‒ Δ 2.0 difference in CAP - 1002 vs. placebo at 6 - months 8 p= 0.0549 p= 0.0299 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in Sept. ITT Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance n=10 n=8 n=8 n=6
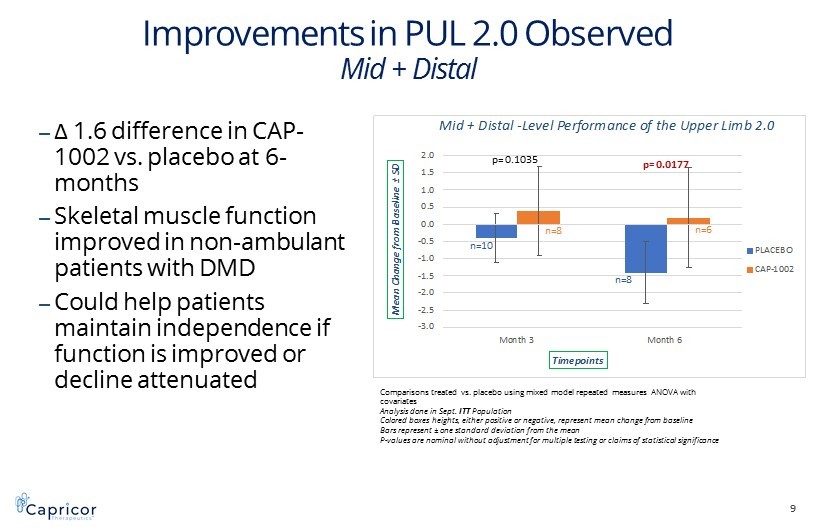
-3.0 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints Mid + Distal -Level Performance of the Upper Limb 2.0 PLACEBO CAP-1002 Improvements in PUL 2.0 Observed Mid + Distal ‒ Δ 1.6 difference in CAP - 1002 vs. placebo at 6 - months ‒ Skeletal muscle function improved in non - ambulant patients with DMD ‒ Could help patients maintain independence if function is improved or decline attenuated 9 p= 0.1035 p= 0.0177 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in Sept. ITT Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance n=10 n=8 n=8 n=6
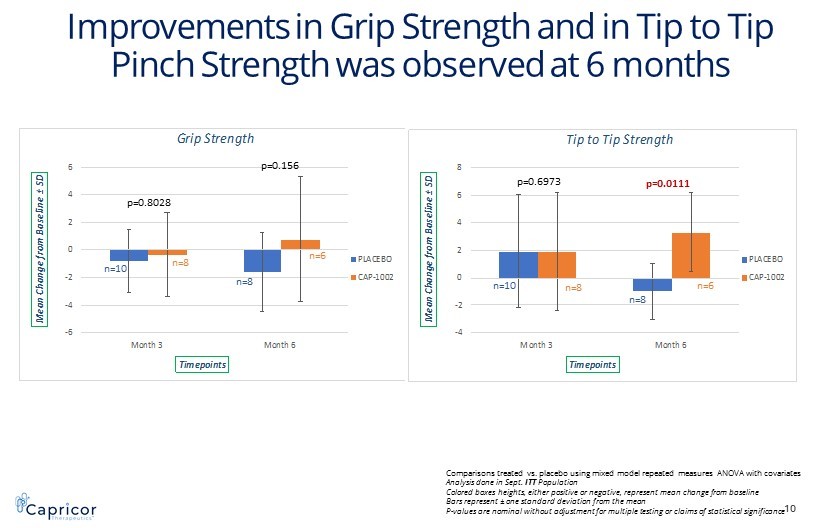
-6 -4 -2 0 2 4 6 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints Grip Strength PLACEBO CAP-1002 -4 -2 0 2 4 6 8 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints Tip to Tip Strength PLACEBO CAP-1002 I mprovements in Grip Strength and in Tip to Tip Pinch Strength was observed at 6 months 10 p=0.8028 p=0.156 p=0.6973 p=0.0111 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in Sept. ITT Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance n=10 n=8 n=8 n=6 n=10 n=8 n=8 n=6
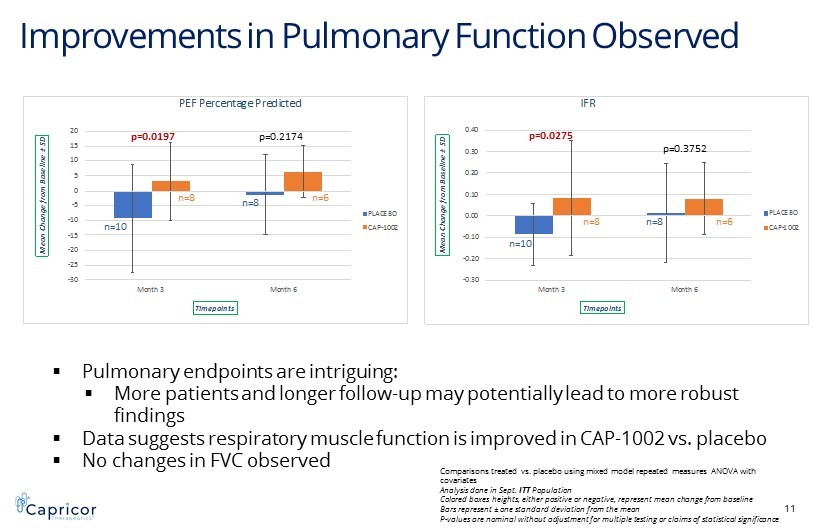
Improvements in Pulmonary Function Observed 11 -30 -25 -20 -15 -10 -5 0 5 10 15 20 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints PEF Percentage Predicted PLACEBO CAP-1002 -0.30 -0.20 -0.10 0.00 0.10 0.20 0.30 0.40 Month 3 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoints IFR PLACEBO CAP-1002 p=0.0197 p=0.2174 n=10 n=8 n=8 n=6 p=0.0275 p=0.3752 n=10 n=8 n=8 n=6 ▪ Pulmonary endpoints are intriguing: ▪ More patients and longer follow - up may potentially lead to more robust findings ▪ Data suggests respiratory muscle function is improved in CAP - 1002 vs. placebo ▪ No changes in FVC observed Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in Sept. ITT Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance
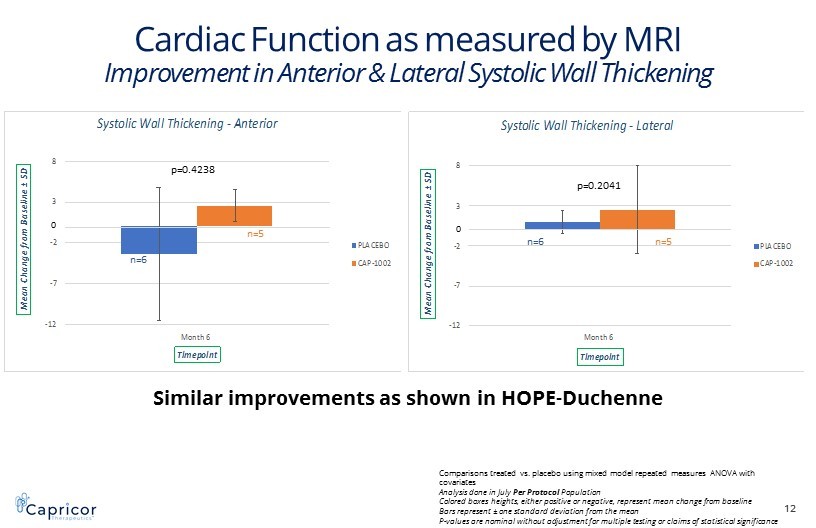
-12 -7 -2 3 8 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoint Systolic Wall Thickening -Anterior PLACEBO CAP-1002 -12 -7 -2 3 8 Month 6 M e a n C h a n g e f r o m B a s e l i n e ± S D Timepoint Systolic Wall Thickening -Lateral PLACEBO CAP-1002 Cardiac Function as measured by MRI Improvement in Anterior & Lateral Systolic Wall Thickening 12 p=0.4238 p=0.2041 Similar improvements as shown in HOPE - Duchenne Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in July Per Protocol Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance n=6 n=5 n=6 n=5 0 0
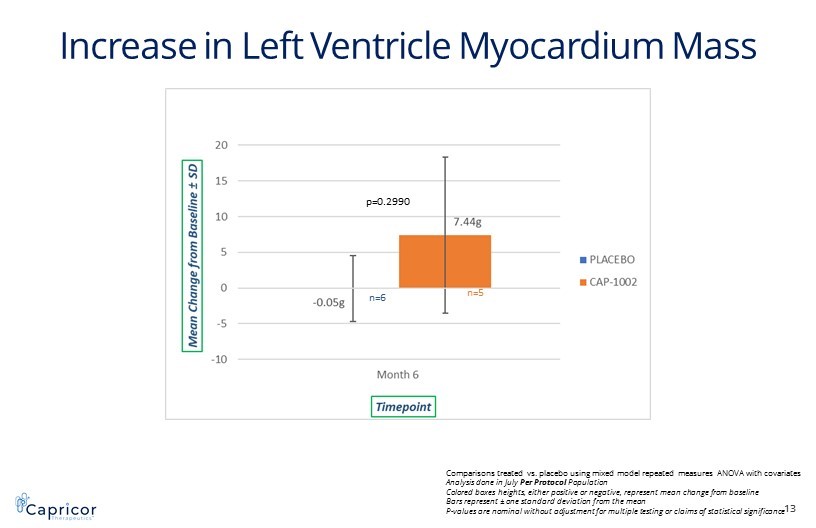
Increase in Left Ventricle Myocardium Mass 13 p=0.2990 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates Analysis done in July Per Protocol Population Colored boxes heights, either positive or negative, represent mean change from baseline Bars represent ± one standard deviation from the mean P - values are nominal without adjustment for multiple testing or claims of statistical significance n=6 n=5
HOPE - 2 Interim Analysis Data Summary 14 ▪ Skeletal: ▪ Mid - Level PUL 2.0 at 6 months (p=0.0612) (ITT) and (p=0.0389) (July PP) ▪ Shoulder + Mid + Distal Level PUL 2.0 at 6 months (p=0.0299) and strong signal at 3 months (p=0.0549) ▪ Mid + Distal Level PUL 2.0 at 6 months (p=0.0177) ▪ Tip to Tip strength (independent skeletal measure) at 6 months (p=0.0111) ▪ Respiratory ▪ Trends towards improvements in PEF (% predicted) and IFR (absolute) ▪ Cardiac ▪ Improvements in wall thickening (similar to positive changes seen in HOPE - Duchenne) ▪ LV myocardium mass
Conclusions and Future Directions Conclusions: ▪ First placebo - controlled trial in DMD to use PUL 2.0 for evaluation of efficacy ▪ First placebo - controlled trial showing upper limb functional improvements in non - ambulant DMD patients ▪ Directionally consistent improvements in function, strength, pulmonary and cardiac endpoints Moving Forward: ▪ Meet with FDA to determine if CAP - 1002 potentially qualifies for accelerated approval based on RMAT standards – Based on Guidance for Industry: Expedited Programs for Regenerative Medicine Therapies for Serious Conditions 15
Acknowledgements ▪ Craig McDonald, MD (UC Davis) ▪ Cuixia Tian, MD (CCHMC) ▪ Russell Butterfield, MD (University of Utah) ▪ Richard Finkel, MD (Nemours Children’s Hospital) ▪ Joanne Janas , MD (Children’s Hospital of Colorado) ▪ Matthew Harmelink, MD (Children’s Hospital of Wisconsin) ▪ Arun Varadhachary, MD (Washington University, Saint Louis Children’s Hospital) ▪ Brenda Wong, MD (University of Massachusetts) ▪ Katherine Mathews, MD (University of Iowa, Children’s Hospital) ▪ All patients and their families who participated in the HOPE - 2 Study ▪ Parent Project Muscular Dystrophy ▪ Coalition Duchenne ▪ CureDuchenne ▪ HOPE - Duchenne was funded with the support of CIRM 16
World - Class DMD Advisory Board Craig McDonald, M.D. (National PI) University of California at Davis (USA) Michelle Eagle, Ph.D., M.Sc., MCSP Atom International Ltd (UK) Pat Furlong Parent Project Muscular Dystrophy (USA) Kan Hor, M.D. Nationwide Children's Hospital (USA) Oscar Henry Mayer, M.D. Children's Hospital of Philadelphia (USA) Eugenio Mercuri, M.D., Ph.D. Catholic University of the Sacred Heart (Italy) Francesco Muntoni, M.D. University College London (UK) Thomas Voit , M.D. University College London (UK) Lee Sweeney, Ph.D. University of Florida (USA) Michael Taylor, M.D., Ph.D. Cincinnati Children's Hospital Medical Center (USA) 17