| Linda Marbán, Ph.D. Chief Executive Officer Capricor Therapeutics, Inc. NASDAQ: CAPR September 24, 2024 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Forward Looking Statements Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor’s product candidates; the initiation, conduct, size, timing and results of discovery efforts and clinical trials; the pace of enrollment of clinical trials; plans regarding regulatory filings, future research and clinical trials; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market; manufacturing capabilities; dates for regulatory meetings; statements about our financial outlook; the ability to achieve product milestones and to receive milestone payments from commercial partners; potential future agreements; plans regarding current and future collaborative activities and the ownership of commercial rights; scope, duration, validity and enforceability of intellectual property rights; future reimbursement prices; future revenue streams and projections; expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings; and any other statements about Capricor’s management team’s future expectations, beliefs, goals, plans or prospects constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words “believes,” “plans,” “could,” “anticipates,” “expects,” “estimates,” “should,” “target,” “will,” “would” and similar expressions) should also be considered to be forward-looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward-looking statements. More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10-K for the year ended December 31, 2023 as filed with the Securities and Exchange Commission on March 11, 2024 and in our Quarterly Report on Form 10-Q for the period ended June 30, 2024 as filed with the Securities and Exchange Commission on August 8, 2024. All forward-looking statements in this press release are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward-looking statements. Deramiocel (CAP-1002) is an Investigational New Drug and is not approved for any indications. None of Capricor’s exosome-based candidates have been approved for clinical investigation. 2 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 3 At Capricor, we stand committed to pushing the boundaries of possibility and forging a path toward transformative treatments for patients in need |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel for the Treatment of DMD Pathway to BLA ➢Capricor intends to submit a BLA for full approval ➢BLA filing supported with Capricor’s existing cardiac and available natural history data ➢Submission will seek broad DMD-cardiomyopathy label ➢If approved, this would serve to address an extensive population of DMD patients (mutation agnostic) ➢This strategy expedites our path to market 4 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 5 Key Takeaways from Pre-BLA Meeting ➢ Strength of Capricor’s long-term cardiac data ➢ Emergence of natural history cardiac dataset ➢ FDA’s continued commitment to rare diseases ➢ No approved therapies for DMD-cardiomyopathy |
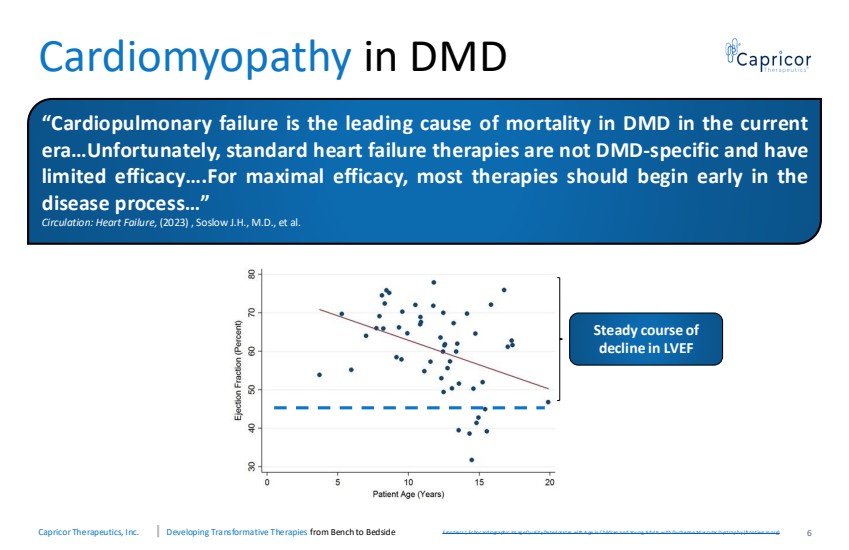
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Cardiomyopathy in DMD F rontiers | Ec hoc ardiogra phic Im ag e Q ual ity Deteri ora tes wi th A ge in Children a nd Young Adul ts with Duchenne M us cular D ystrophy (frontiers in.org) Steady course of decline in LVEF 6 “Cardiopulmonary failure is the leading cause of mortality in DMD in the current era…Unfortunately, standard heart failure therapies are not DMD-specific and have limited efficacy….For maximal efficacy, most therapies should begin early in the disease process…” Circulation: Heart Failure, (2023) , Soslow J.H., M.D., et al. |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 7 • Deramiocel(CAP-1002): biologic consisting of allogeneic cardiosphere-derived cells (CDCs) • Multiple-modalities • Immunomodulatory • Anti-inflammatory • Anti-fibrotic • Pro-angiogenic • Investigated in over 200 patients • Potency assays accepted by FDA which support MOA • FDA designations in DMD ✓ Orphan Drug Designation ✓ Regenerative Medicine Advanced Therapy (RMAT) designation ✓ Rare Pediatric Disease Designation • Capricor holds full rights to the PRV, if received Deramiocel Cell Therapy Overview |
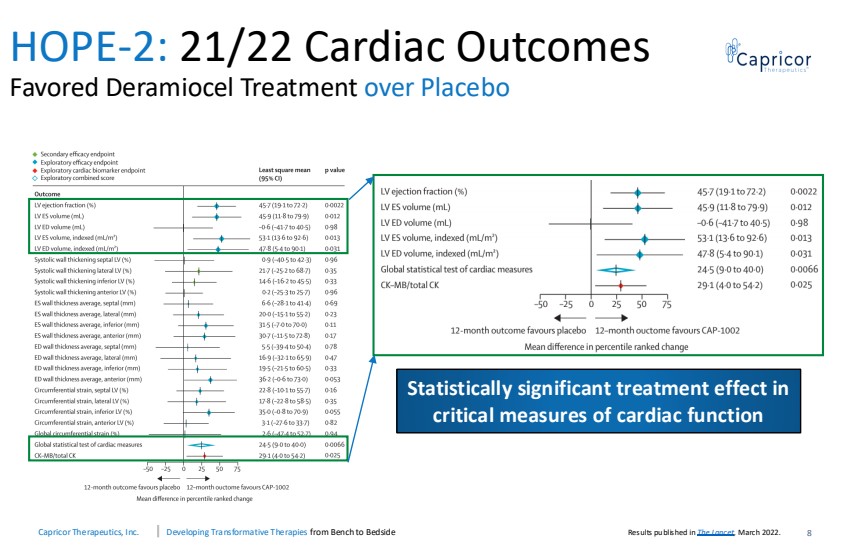
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 8 HOPE-2: 21/22 Cardiac Outcomes Favored Deramiocel Treatment over Placebo Statistically significant treatment effect in critical measures of cardiac function Results published in The Lancet, March 2022. |
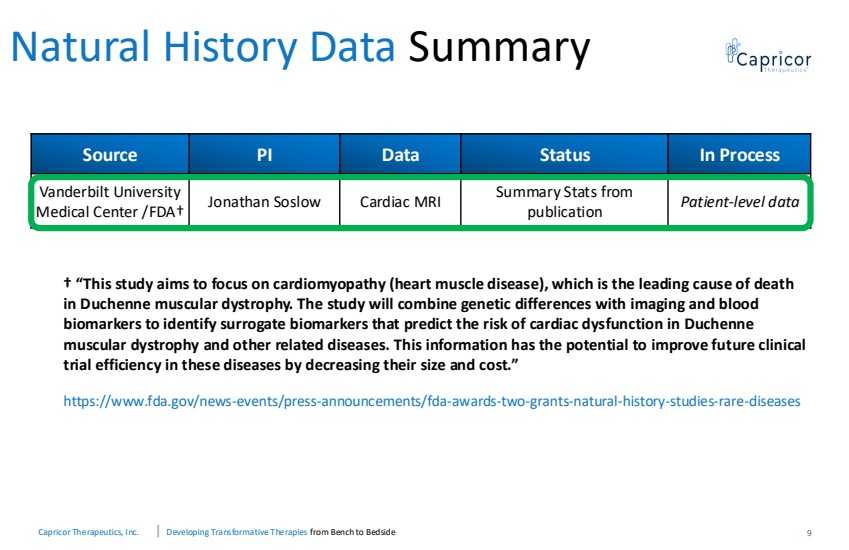
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 9 Natural History Data Summary † “This study aims to focus on cardiomyopathy (heart muscle disease), which is the leading cause of death in Duchenne muscular dystrophy. The study will combine genetic differences with imaging and blood biomarkers to identify surrogate biomarkers that predict the risk of cardiac dysfunction in Duchenne muscular dystrophy and other related diseases. This information has the potential to improve future clinical trial efficiency in these diseases by decreasing their size and cost.” https://www.fda.gov/news-events/press-announcements/fda-awards-two-grants-natural-history-studies-rare-diseases Source PI Data Status In Process Vanderbilt University Medical Center /FDA† Jonathan Soslow Cardiac MRI Summary Stats from publication Patient-level data |
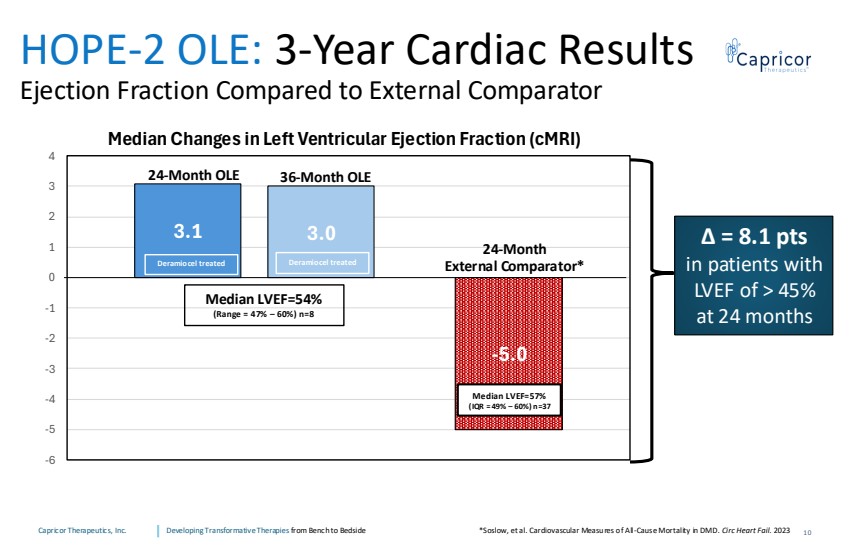
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 3.0 -5.0 3.1 -6 -5 -4 -3 -2 -1 0 1 2 3 4 Median Changes in Left Ventricular Ejection Fraction (cMRI) Median LVEF=54% (Range = 47% – 60%) n=8 Deramiocel treated Deramiocel treated HOPE-2 OLE: 3-Year Cardiac Results Ejection Fraction Compared to External Comparator 24-Month External Comparator* 24-Month OLE 36-Month OLE ∆ = 8.1 pts in patients with LVEF of > 45% at 24 months Median LVEF=57% (IQR = 49% – 60%) n=37 10 *Soslow, et al. Cardiovascular Measures of All-Cause Mortality in DMD. Circ Heart Fail. 2023 |
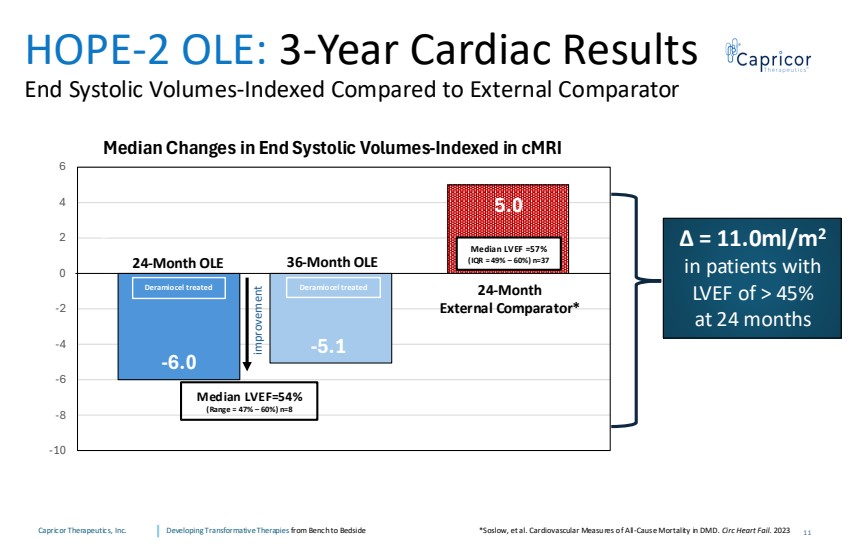
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside -5.1 5.0 -6.0 -10 -8 -6 -4 -2 0 2 4 6 Median Changes in End Systolic Volumes-Indexed in cMRI Median LVEF =57% (IQR = 49% – 60%) n=37 Median LVEF=54% (Range = 47% – 60%) n=8 HOPE-2 OLE: 3-Year Cardiac Results End Systolic Volumes-Indexed Compared to External Comparator 24-Month External Comparator* 24-Month OLE 36-Month OLE T IMPROVEMEN ∆ = 11.0ml/m2 in patients with LVEF of > 45% at 24 months 11 *Soslow, et al. Cardiovascular Measures of All-Cause Mortality in DMD. Circ Heart Fail. 2023 improvement Deramiocel treated Deramiocel treated |
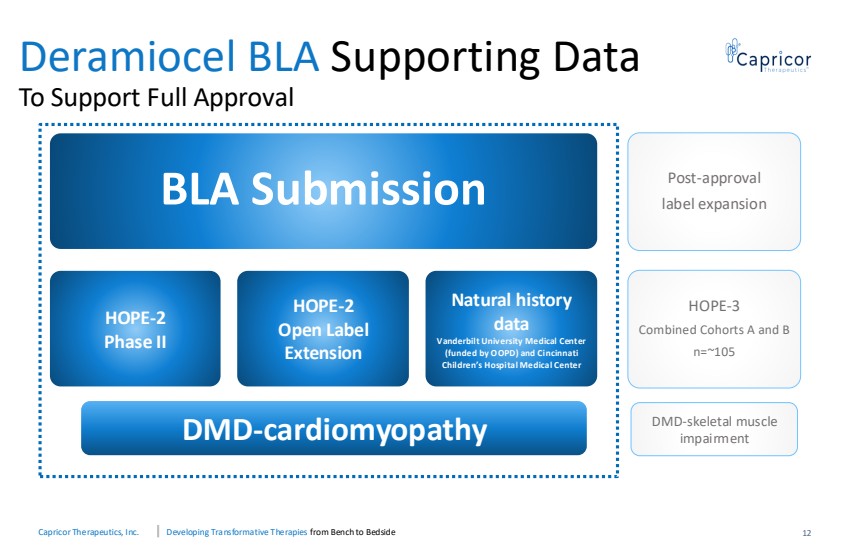
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel BLA Supporting Data To Support Full Approval 12 BLA Submission HOPE-2 Phase II HOPE-2 Open Label Extension Natural history data Vanderbilt University Medical Center (funded by OOPD) and Cincinnati Children’s Hospital Medical Center Post-approval label expansion HOPE-3 Combined Cohorts A and B n=~105 DMD-cardiomyopathy DMD-skeletal muscle impairment |
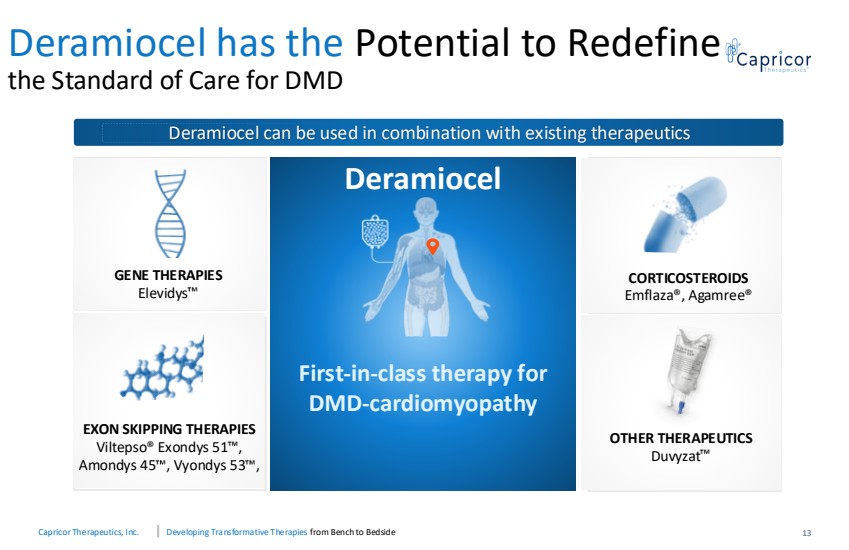
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel has the Potential to Redefine the Standard of Care for DMD 13 Deramiocel First-in-class therapy for DMD-cardiomyopathy GENE THERAPIES Elevidys EXON SKIPPING THERAPIES Viltepso® Exondys 51 , Amondys 45 , Vyondys 53 , CORTICOSTEROIDS Emflaza®, Agamree® OTHER THERAPEUTICS Duvyzat Deramiocel can be used in combination with existing therapeutics |
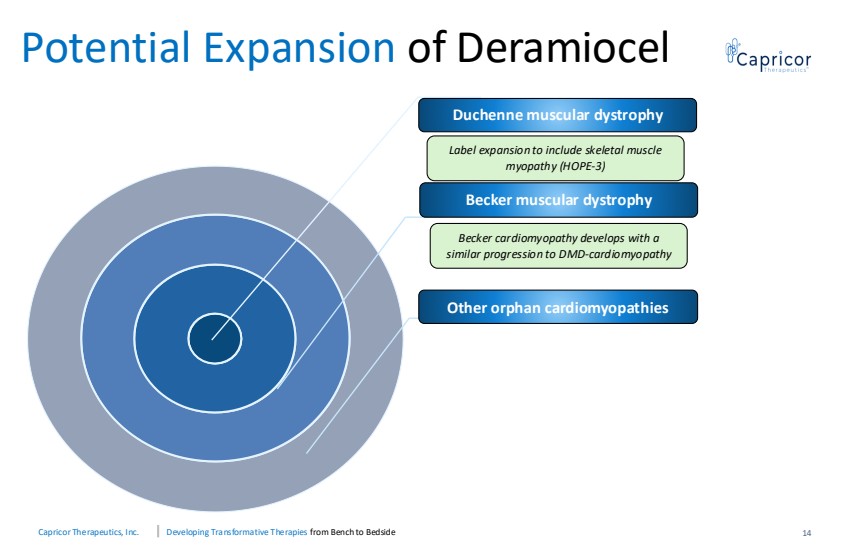
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Potential Expansion of Deramiocel Becker cardiomyopathy develops with a similar progression to DMD-cardiomyopathy Duchenne muscular dystrophy Becker muscular dystrophy Other orphan cardiomyopathies 14 Label expansion to include skeletal muscle myopathy (HOPE-3) |
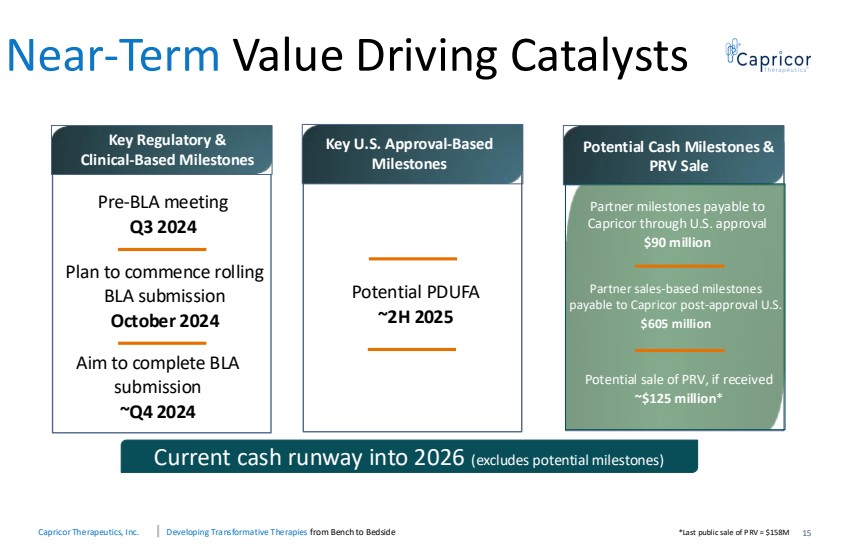
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Near-Term Value Driving Catalysts 15 Aim to complete BLA submission ~Q4 2024 Key Regulatory & Clinical-Based Milestones Pre-BLA meeting Q3 2024 Plan to commence rolling BLA submission October 2024 Key U.S. Approval-Based Milestones Potential PDUFA ~2H 2025 Potential Cash Milestones & PRV Sale Partner milestones payable to Capricor through U.S. approval $90 million Partner sales-based milestones payable to Capricor post-approval U.S. $605 million Potential sale of PRV, if received ~$125 million* *Last public sale of PRV = $158M Current cash runway into 2026 (excludes potential milestones) |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 16 Capricor Therapeutics, Inc. 10865 Road to the Cure, Suite 150 San Diego, California 92121 e: w: info@capricor.com www.capricor.com Nasdaq: CAPR Thank you Q&A |